Exam 9: Covalent Bonding: Orbitals
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
The hybridization of the central atom in ClF2+ is:
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
Which of the following has the shortest bond length?
Free
(Multiple Choice)
4.8/5  (47)
(47)
Correct Answer:
D
The hybridization of the carbon atom in the cation CH3+ is:
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
A
For how many of the following does bond order decrease if you take away one electron from the neutral molecule? B2,C2,P2,F2
(Multiple Choice)
4.8/5  (45)
(45)
Use the molecules below to answer the next three questions. 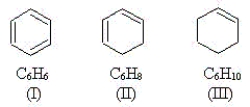 -Which molecule(s)have equivalent C-C bonds throughout the molecule?
-Which molecule(s)have equivalent C-C bonds throughout the molecule?
(Multiple Choice)
4.7/5  (44)
(44)
Which of the following statements about the molecule BN is false?
(Multiple Choice)
4.9/5  (47)
(47)
How many electrons are involved in pi bonding in benzene,C6H6?
(Multiple Choice)
4.8/5  (33)
(33)
__________ causes a substance to be attracted into the inducing magnetic field.
(Short Answer)
4.8/5  (30)
(30)
The number of molecular orbitals formed is always __________ the number of atomic orbitals combined.
(Short Answer)
4.8/5  (26)
(26)
Sulfur trioxide is known to be planar with all the oxygen atoms equidistant from the central sulfur atom.On the basis of these facts,which of the following conclusions may be drawn concerning this molecule? I.It can be represented by three equivalent resonance structures.
II.The dipoles associated with each S-O bond are equal in magnitude.
III.The sulfur atom is sp2 hybridized.
(Multiple Choice)
5.0/5  (42)
(42)
Tetracyanoethylene has the skeleton shown below: 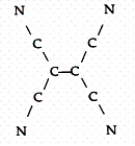 From its Lewis structure determine the following:
-How many sigma and pi bonds are in the molecule?
From its Lewis structure determine the following:
-How many sigma and pi bonds are in the molecule?
(Multiple Choice)
4.8/5  (30)
(30)
Tetracyanoethylene has the skeleton shown below:  From its Lewis structure determine the following:
-How many of the atoms are sp2 hybridized?
From its Lewis structure determine the following:
-How many of the atoms are sp2 hybridized?
(Multiple Choice)
4.7/5  (33)
(33)
Consider three molecules - A,B,C.Molecule A has a hybridization of sp3.Molecule B has two more effective pairs (electron pairs around the central atom)than molecule A.Molecule C consists of one bond and two bonds.Give the molecular structure,hybridization,bond angles,and an example for each molecule.
(Essay)
4.8/5  (38)
(38)
How many unpaired electrons in the F22+ ion are based on molecular orbital theory? The order of the molecular orbitals are ( 2s)( *2s)( 2p)( 2p)( *2p)( *2p).
(Multiple Choice)
4.7/5  (40)
(40)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)