Deck 21: Transition Metals and Coordination Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/142
Play
Full screen (f)
Deck 21: Transition Metals and Coordination Chemistry
1
Transition metals display great similarities
A)within a given period
B)within a given vertical group
C)with the semimetals
D)all of these
E)A and B only
A)within a given period
B)within a given vertical group
C)with the semimetals
D)all of these
E)A and B only
A and B only
2
The phenomenon called __________ contraction is responsible for the great similarity in atomic size and chemistry of 4d and 5d elements.
A)transition
B)coordination
C)lanthanide
D)isomeric
E)none of these
A)transition
B)coordination
C)lanthanide
D)isomeric
E)none of these
lanthanide
3
The electron configuration of Ti2+ is
A)[Ar] 4s2
B)[Ar] 4s13d1
C)[Ar] 3d2
D)[Ar] 4s23d2
E)none of these
A)[Ar] 4s2
B)[Ar] 4s13d1
C)[Ar] 3d2
D)[Ar] 4s23d2
E)none of these
[Ar] 3d2
4
What is the electron configuration of the Sc(I)ion?
A)[Ar] 4s14d1
B)[Ar] 4s13d1
C)[Ar] 3s13d1
D)[Ar] 4s2
E)[Ar] 3d2
A)[Ar] 4s14d1
B)[Ar] 4s13d1
C)[Ar] 3s13d1
D)[Ar] 4s2
E)[Ar] 3d2

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
5
Which metal ion has a d5 electron configuration?
A)Cr2+
B)Ag+
C)Mn2+
D)Os2+
E)Co2+
A)Cr2+
B)Ag+
C)Mn2+
D)Os2+
E)Co2+

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
6
What is the electron configuration of the Mn(II)ion?
A)[Ar] 4s23d5
B)[Ar] 4s13d5
C)[Ar] 4s23d3
D)[Ar] 3d5
E)none of these
A)[Ar] 4s23d5
B)[Ar] 4s13d5
C)[Ar] 4s23d3
D)[Ar] 3d5
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is true?
A)The first ionization energy for Zn is significantly higher than that of Sc.
B)The first ionization energy for Zn is significantly lower than that of Sc.
C)The third ionization energy for Zn is significantly higher than that of Sc.
D)The third ionization energy for Zn is significantly lower than that of Sc.
E)Two of these are correct.
A)The first ionization energy for Zn is significantly higher than that of Sc.
B)The first ionization energy for Zn is significantly lower than that of Sc.
C)The third ionization energy for Zn is significantly higher than that of Sc.
D)The third ionization energy for Zn is significantly lower than that of Sc.
E)Two of these are correct.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
8
A complex ion is a charged species consisting of a metal ion surrounded by
A)other transition metals
B)hydrogen ions
C)ligands
D)ligands and counter ions
E)none of these
A)other transition metals
B)hydrogen ions
C)ligands
D)ligands and counter ions
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
9
The electron configuration for Cr2+ is
A)[Ar] 4s23d4
B)[Ar] 4s13d5
C)[Ar] 3d4
D)[Ar] 4s23d2
E)none of these
A)[Ar] 4s23d4
B)[Ar] 4s13d5
C)[Ar] 3d4
D)[Ar] 4s23d2
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following transition metals are important to the U.S.economy and defense?
A)chromium and cobalt
B)manganese
C)platinum and palladium
D)all of these
E)A and B only
A)chromium and cobalt
B)manganese
C)platinum and palladium
D)all of these
E)A and B only

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the transition metals is the best conductor of heat and electric current?
A)copper
B)silver
C)gold
D)tungsten
E)titanium
A)copper
B)silver
C)gold
D)tungsten
E)titanium

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
12
How many ligands are in this representative drawing of a complex ion? 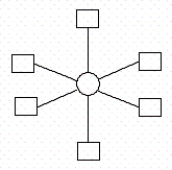
A)1
B)3
C)6
D)7
E)8

A)1
B)3
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
13
Why do transition metals show a lot of chemical similarities within a given period?
A)The valence s and p electrons affect their chemistry more so than the inner d and f electrons,which do not participate in bonding as easily.
B)The number of electrons within a given period varies only slightly and is sometimes identical because these metals have more than one ionic form.
C)All elements in a given period,including the representative elements,have a lot of chemical similarities due to the gradual increase in atomic number.
D)The transition metals always fill their s and p orbitals first before filling their d orbitals,which affects their chemistry.
E)None of the above is correct.
A)The valence s and p electrons affect their chemistry more so than the inner d and f electrons,which do not participate in bonding as easily.
B)The number of electrons within a given period varies only slightly and is sometimes identical because these metals have more than one ionic form.
C)All elements in a given period,including the representative elements,have a lot of chemical similarities due to the gradual increase in atomic number.
D)The transition metals always fill their s and p orbitals first before filling their d orbitals,which affects their chemistry.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is incorrect concerning the 3d,4d,and 5d transition series?
A)There is a significant increase in radius in going from the 3d to the 4d metals,but the 4d and 5d metals are similar in size.
B)There is a general decrease in size going from left to right for each of these series due to the increasing nuclear charge.
C)The separation of hafnium and zirconium found together in nature is difficult due to their similarities in chemistry,which is attributed to their virtually identical sizes.
D)Cerium through lutetium exhibits what is referred to as the lanthanide contraction.
E)All of the above are correct.
A)There is a significant increase in radius in going from the 3d to the 4d metals,but the 4d and 5d metals are similar in size.
B)There is a general decrease in size going from left to right for each of these series due to the increasing nuclear charge.
C)The separation of hafnium and zirconium found together in nature is difficult due to their similarities in chemistry,which is attributed to their virtually identical sizes.
D)Cerium through lutetium exhibits what is referred to as the lanthanide contraction.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following transition metals is most likely to form an oxide?
A)gold
B)silver
C)platinum
D)palladium
E)copper
A)gold
B)silver
C)platinum
D)palladium
E)copper

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
16
What is the electron configuration of the Ni(II)ion?
A)[Ar] 4s23d6
B)[Ar] 4s13d7
C)[Ar] 4s23d8
D)[Ar] 3d8
E)none of these
A)[Ar] 4s23d6
B)[Ar] 4s13d7
C)[Ar] 4s23d8
D)[Ar] 3d8
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
17
The electron configuration of Cr3+ is
A)[Ar] 4s23d1
B)[Ar] 4s13d2
C)[Ar] 3d3
D)[Ar] 4s23d4
E)none of these
A)[Ar] 4s23d1
B)[Ar] 4s13d2
C)[Ar] 3d3
D)[Ar] 4s23d4
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a d7 ion?
A)Co(II)
B)Cu(II)
C)Mn(II)
D)Mn(IV)
E)At least two of the above (a-d)are d7 ions.
A)Co(II)
B)Cu(II)
C)Mn(II)
D)Mn(IV)
E)At least two of the above (a-d)are d7 ions.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
19
What is the maximum oxidation state expected for manganese?
A)+2
B)+8
C)+6
D)+5
E)+7
A)+2
B)+8
C)+6
D)+5
E)+7

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
20
The reducing abilities of the first-row transition metals generally __________ going from left to right across the period.
A)decrease
B)increase
C)stay the same
D)none of these
E)remain at 1.0 V
A)decrease
B)increase
C)stay the same
D)none of these
E)remain at 1.0 V

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
21
Co2+ in water is blue.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
22
What transition metal is used in magnets,catalysts,and drill bits?
A)nickel
B)copper
C)platinum
D)cobalt
E)titanium
A)nickel
B)copper
C)platinum
D)cobalt
E)titanium

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
23
How many d electrons are present on the metal ion in the complex ion PtCl62-?
A)8
B)6
C)4
D)3
E)2
A)8
B)6
C)4
D)3
E)2

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
24
What are the oxidation numbers of each central metal atom in the following coordination compounds? K3[Fe(CN)6],[Cr(NH3)4Br2]Br,[Ni(H2O)6]Cl2,Na2[TaF7]
A)3,3,3,5
B)3,3,2,7
C)-3,3,2,5
D)-3,1,2,5
E)3,3,2,5
A)3,3,3,5
B)3,3,2,7
C)-3,3,2,5
D)-3,1,2,5
E)3,3,2,5

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
25
This transition metal is used in the production of a hard steel used for rock crushers and bank vaults,and can be found in nodules on the ocean floor.
A)iron
B)manganese
C)magnesium
D)cobalt
E)nickel
A)iron
B)manganese
C)magnesium
D)cobalt
E)nickel

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
26
Which 3d transition metal is mixed with concentrated sulfuric acid to give a powerful cleaning solution for removing organic materials from analytical glassware?
A)chromium
B)iron
C)cobalt
D)scandium
E)manganese
A)chromium
B)iron
C)cobalt
D)scandium
E)manganese

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
27
What transition metal has the combination of toughness,stretchability,and resilience that makes it ideal for use in bicycle frames?
A)titanium
B)platinum
C)tungsten
D)nickel
E)aluminum
A)titanium
B)platinum
C)tungsten
D)nickel
E)aluminum

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
28
The election configuration of Mn2+ is [Ar] 4s23d3.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
29
What heavy metal is the most abundant and most important to our civilization?
A)iron
B)gold
C)magnesium
D)cobalt
E)copper
A)iron
B)gold
C)magnesium
D)cobalt
E)copper

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the metal ions in the following complex ions has a d5 electron configuration?
A)V(H2O)62+
B)Mo(NH3)63+
C)Co(CN)4-
D)Fe(CN)63-
E)RhCl64-
A)V(H2O)62+
B)Mo(NH3)63+
C)Co(CN)4-
D)Fe(CN)63-
E)RhCl64-

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
31
The expected electron configuration of Cu+ is [Ar] 3s13d9.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
32
An element that is a significant component of both brass and bronze is:
A)nickel
B)tin
C)copper
D)iron
E)zinc
A)nickel
B)tin
C)copper
D)iron
E)zinc

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
33
The metals with the highest ionization energies are most likely to be found in nature in the elemental state.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
34
Which transition metal is valued for its high electrical conductivity and resistance to corrosion,and is widely used for plumbing?
A)iron
B)manganese
C)magnesium
D)cobalt
E)copper
A)iron
B)manganese
C)magnesium
D)cobalt
E)copper

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
35
What transition metal is mainly used for galvanizing steel?
A)iron
B)zinc
C)copper
D)cobalt
E)nickel
A)iron
B)zinc
C)copper
D)cobalt
E)nickel

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
36
The coordination theory was proposed by:
A)Bailar
B)Jorgensen
C)Blomstrand
D)Werner
E)none of these
A)Bailar
B)Jorgensen
C)Blomstrand
D)Werner
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
37
True or false: Transition metals show great similarities both within a given period and within a given vertical group.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
38
Which metal is most widely used in the electrical systems of homes and factories?
A)copper
B)silver
C)gold
D)tungsten
E)titanium
A)copper
B)silver
C)gold
D)tungsten
E)titanium

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
39
Metals usually have higher melting points than nonmetals.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
40
What transition metal is used in stainless steel?
A)nickel
B)titanium
C)chromium
D)iridium
E)niobium
A)nickel
B)titanium
C)chromium
D)iridium
E)niobium

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
41
Because they have the same atoms,bonds,and formulas,geometrical isomers have the same color.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
42
In which of the following complexes does the transition metal have a d8 configuration?
A)PtCl42-
B)Cu(H2O)62+
C)Ni(CO)4
D)Zn(NH3)42+
E)Fe(CN)63-
A)PtCl42-
B)Cu(H2O)62+
C)Ni(CO)4
D)Zn(NH3)42+
E)Fe(CN)63-

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula for the hexaaquachromium(II)ion?
A)[Cr(H2O)6]2+
B)[Cr2(H2O)6]4+
C)[Cr(H2O)4]2+
D)[Cr2(H2O)6]2+
E)[Cr(H2O)6]4-
A)[Cr(H2O)6]2+
B)[Cr2(H2O)6]4+
C)[Cr(H2O)4]2+
D)[Cr2(H2O)6]2+
E)[Cr(H2O)6]4-

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
44
Suppose you are studying coordination compounds of Co(II)with the ligand pyridine (py,C5H5N,molar mass = 79.10).You isolate a crystalline compound,and since the only available anions are Cl- and NO3-,you hypothesize the empirical formula of the coordination compound must be Cow(py)x(Cl)y(NO3)z.
You analyze for pyridine (Kb is approximately 10-9)by dissolving 0.1000 g of complex in 10 mL of H2O and titrating with a 0.01 M HCl solution.Which of the following indicators should be used to detect the endpoint? (Assume that the initial concentration of pyridine is approximately 0.01 M. )
A)bromophenol blue,pH range of color change = 3.0-4.6
B)methyl red,pH range of color change = 4.8-6.0
C)bromothymol blue,pH range of color change = 6.0-7.6
D)thymol blue,pH range of color change = 8.0 -9.6
E)alizarin yellow,pH range of color change = 10.1-12.0
You analyze for pyridine (Kb is approximately 10-9)by dissolving 0.1000 g of complex in 10 mL of H2O and titrating with a 0.01 M HCl solution.Which of the following indicators should be used to detect the endpoint? (Assume that the initial concentration of pyridine is approximately 0.01 M. )
A)bromophenol blue,pH range of color change = 3.0-4.6
B)methyl red,pH range of color change = 4.8-6.0
C)bromothymol blue,pH range of color change = 6.0-7.6
D)thymol blue,pH range of color change = 8.0 -9.6
E)alizarin yellow,pH range of color change = 10.1-12.0

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
45
Ethylenediamine (en)is a bidentate ligand.What is the coordination number of cobalt in [Co(en)2Cl2]Cl?
A)four
B)five
C)seven
D)eight
E)six
A)four
B)five
C)seven
D)eight
E)six

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
46
When 6.3 moles of [Co(NH3)5Cl]Cl2 is dissolved in water,how many moles of ions are in solution?
A)3.3
B)4.3
C)2.1
D)19
E)57
A)3.3
B)4.3
C)2.1
D)19
E)57

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
47
Analysis of the data from a titration indicates that a 0.1190-g sample of the complex contains 0.0844 g of py.Further analysis shows that 0.1190 g of the complex contains a 0.0157 g of cobalt and 0.0189 g of chloride.What is the empirical formula of the complex?
A)Co(py)6(Cl)(NO3)
B)Co(py)4Cl2
C)Co2(py)5(Cl)2(NO3)2
D)Co3(py)8(Cl)2(NO3)4
E)Co(py)4(NO3)2
A)Co(py)6(Cl)(NO3)
B)Co(py)4Cl2
C)Co2(py)5(Cl)2(NO3)2
D)Co3(py)8(Cl)2(NO3)4
E)Co(py)4(NO3)2

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3?
A)[Cr(NH3)3Cl3]
B)[Cr(NH3)6]Cl3
C)[Cr(NH3)Cl]NO3
D)Na3[Cr(CN)6]
E)Na3[CrCl6]
A)[Cr(NH3)3Cl3]
B)[Cr(NH3)6]Cl3
C)[Cr(NH3)Cl]NO3
D)Na3[Cr(CN)6]
E)Na3[CrCl6]

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following complexes can exhibit optical isomerism? (en = H2N-CH2-CH2-NH2 and is bidentate)
A)cis-Co(NH3)4Cl2
B)trans-Co(en)2Br2
C)cis-Co(en)2Cl2
D)Co(NH3)3Cl3
E)none of these
A)cis-Co(NH3)4Cl2
B)trans-Co(en)2Br2
C)cis-Co(en)2Cl2
D)Co(NH3)3Cl3
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
50
What is the correct IUPAC name for [AuCl4(H2O)2]-?
A)diaquatetrachloroaurate(0)ion
B)diaquatetrachloroaurate(III)ion
C)diaquatetrachloroaurate(I)ion
D)diaquatetrachlorogold(III)ion
E)diaquatetrachlorogold(I)ion
A)diaquatetrachloroaurate(0)ion
B)diaquatetrachloroaurate(III)ion
C)diaquatetrachloroaurate(I)ion
D)diaquatetrachlorogold(III)ion
E)diaquatetrachlorogold(I)ion

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
51
The coordination theory was proposed by Alfred Packer.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
52
What is the sum of all isomers (geometrical and optical)that the complex ion Co(en)2Cl2+ exhibits?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following complexes:
I.Pt(NH3)2Cl2
(square planar)
II.Rh(en)32+
(en = H2N-CH2-CH2-NH2 and is bidentate)
III.CoCl42-
(tetrahedral)
Which can exhibit cis-trans isomerism?
A)I
B)II
C)III
D)I,II
E)I,II,III
I.Pt(NH3)2Cl2
(square planar)
II.Rh(en)32+
(en = H2N-CH2-CH2-NH2 and is bidentate)
III.CoCl42-
(tetrahedral)
Which can exhibit cis-trans isomerism?
A)I
B)II
C)III
D)I,II
E)I,II,III

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
54
A coordination compound of Cu2+ can be described as Cu(NH3)xSO4 and is known to contain 29.92% NH3 by mass.The value of x is:
A)2
B)3
C)4
D)6
E)none of these
A)2
B)3
C)4
D)6
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
55
Suppose you are studying coordination compounds of Co(II)with the ligand pyridine (py,C5H5N,molar mass = 79.10).You isolate a crystalline compound,and since the only available anions are Cl- and NO3-,you hypothesize the empirical formula of the coordination compound must be Cow(py)x(Cl)y(NO3)z.
You discover that the complex decomposes in water.You dissolve 0.1000 g of the complex in H2O and add excess NaHg(SCN)4,which precipitates Co(II)as CoHg(SCN)4(s).After the precipitate is washed and dried,its mass is 0.1102 g.How many grams of cobalt are contained in 0.1000 g of the complex?
A)0.1102
B)0.0396
C)0.0132
D)0.437
E)0.0548
You discover that the complex decomposes in water.You dissolve 0.1000 g of the complex in H2O and add excess NaHg(SCN)4,which precipitates Co(II)as CoHg(SCN)4(s).After the precipitate is washed and dried,its mass is 0.1102 g.How many grams of cobalt are contained in 0.1000 g of the complex?
A)0.1102
B)0.0396
C)0.0132
D)0.437
E)0.0548

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
56
What is the correct formula for sodium tetrachlorocuprate(II)?
A)Na2(CuCl6)
B)Na4(CuCl4)
C)Na(CuCl4)
D)Na2(CuCl4)
E)Na3(CuCl4)
A)Na2(CuCl6)
B)Na4(CuCl4)
C)Na(CuCl4)
D)Na2(CuCl4)
E)Na3(CuCl4)

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
57
The empirical formula of a compound with a mass percent composition of 6.78% H,31.43% N,39.76% Cl,and 22.03% Co is consistent with which of the following complexes?
A)[Co(NH3)3Cl3]
B)[Co(NH3)4Cl2]Cl
C)[Co(NH3)5Cl]Cl2
D)[Co(NH3)6]Cl3
E)none of these
A)[Co(NH3)3Cl3]
B)[Co(NH3)4Cl2]Cl
C)[Co(NH3)5Cl]Cl2
D)[Co(NH3)6]Cl3
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following complexes shows geometrical isomerism?
A)[Co(NH3)5Cl]SO4
B)[Co(NH3)6]Cl3
C)[Co(NH3)5Cl]Cl2
D)K[Co(NH3)2Cl4]
E)none of these
A)[Co(NH3)5Cl]SO4
B)[Co(NH3)6]Cl3
C)[Co(NH3)5Cl]Cl2
D)K[Co(NH3)2Cl4]
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
59
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate,presumably AgCl.You dissolve 0.1000 g of the complex in H2O and perform a precipitation titration with 0.0500 M AgNO3 as the titrant.Using an electrode that is sensitive to [Ag+],you reach the endpoint after 9.00 mL of titrant are added.How many grams of chloride ion were present in the 0.1000-g sample?
A)4.50 10-4
B)5.00 10-3
C)1.77 10-3
D)6.38 10-2
E)1.60 10-2
A)4.50 10-4
B)5.00 10-3
C)1.77 10-3
D)6.38 10-2
E)1.60 10-2

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
60
What is the formula for the pentaamminehydroxocopper(II)ion?
A)[Cu(NH3)(OH)5]3-
B)[Cu(NH3)5(OH)5]2+
C)[Cu(NH3)5(OH)]2+
D)[Cu(NH3)5(OH)5]3-
E)[Cu(NH3)5(OH)]+
A)[Cu(NH3)(OH)5]3-
B)[Cu(NH3)5(OH)5]2+
C)[Cu(NH3)5(OH)]2+
D)[Cu(NH3)5(OH)5]3-
E)[Cu(NH3)5(OH)]+

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
61
The complex FeL62+,where L is a neutral ligand,is known to be diamagnetic.The number of d electrons in this complex ion is:
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
62
NiCl42- (tetrahedral)
A)0
B)1
C)2
D)4
E)5
A)0
B)1
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
63
The complex ions of Zn2+ are all colorless.The most likely explanation for this is:
A)Zn2+ is paramagnetic.
B)Zn2+ exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
C)Since Zn2+ is a d10 ion,it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
D)Zn2+ is not a transition metal ion.
E)None of these is correct.
A)Zn2+ is paramagnetic.
B)Zn2+ exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
C)Since Zn2+ is a d10 ion,it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
D)Zn2+ is not a transition metal ion.
E)None of these is correct.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
64
CoF63- (weak field)
A)0
B)1
C)2
D)4
E)5
A)0
B)1
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
65
The ____ isomer of the complex Ni(en)2Cl2 exhibits optical isomers,but the _____ isomer does not.
A)cis,trans
B)trans,cis
C)Both isomers exhibit optical isomers.
D)Neither isomers exhibit optical isomers.
E)Depends on the wavelength of plane-polarized light used.
A)cis,trans
B)trans,cis
C)Both isomers exhibit optical isomers.
D)Neither isomers exhibit optical isomers.
E)Depends on the wavelength of plane-polarized light used.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
66
The geometry of a coordination compound with a coordination number of 4 is
A)tetrahedral,in order to minimize repulsions between the ligands
B)octahedral,since there are two different positions possible for each ligand
C)square planar,to allow room for the counterion because the ligands take up so much space
D)linear,since there are two ligands on each side of the transition metal
E)tetrahedral or square planar,but too difficult to predict based on the information given
A)tetrahedral,in order to minimize repulsions between the ligands
B)octahedral,since there are two different positions possible for each ligand
C)square planar,to allow room for the counterion because the ligands take up so much space
D)linear,since there are two ligands on each side of the transition metal
E)tetrahedral or square planar,but too difficult to predict based on the information given

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
67
How many of the following compounds exhibit geometric isomers?
I.Pt(NH3)2Cl2 (square planar)
II.[Co(H2O)2]Cl3
III.Ni(NH3)4(NO2)2
IV.K2[CoCl4]
A)0
B)1
C)2
D)3
E)4
I.Pt(NH3)2Cl2 (square planar)
II.[Co(H2O)2]Cl3
III.Ni(NH3)4(NO2)2
IV.K2[CoCl4]
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
68
Copper(I)complexes would be expected to be colorless.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
69
Which complex ion shape is not capable of showing cis-trans isomerism?
A)octahedral
B)square planar
C)tetrahedral
D)two of these
E)none of these
A)octahedral
B)square planar
C)tetrahedral
D)two of these
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements is true about the octahedral complexes of Ni2+?
A)Both strong- and weak-field complexes are diamagnetic.
B)The strong-field complex is diamagnetic and the weak-field complex is paramagnetic.
C)The strong-field complex is paramagnetic and the weak-field complex is diamagnetic.
D)Both strong- and weak-field complexes are paramagnetic.
E)There are no octahedral complexes of Ni.
A)Both strong- and weak-field complexes are diamagnetic.
B)The strong-field complex is diamagnetic and the weak-field complex is paramagnetic.
C)The strong-field complex is paramagnetic and the weak-field complex is diamagnetic.
D)Both strong- and weak-field complexes are paramagnetic.
E)There are no octahedral complexes of Ni.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following statements concerning the complex ion Co(en)2Cl2+ is true? (en = ethylenediamine,NH2CH2CH2NH2)?
A)The complex ion contains Co(I).
B)The complex ion exhibits cis and trans geometrical isomers,but no optical isomers.
C)The complex ion exhibits two geometrical isomers (cis and trans)and two optical isomers.
D)Since en is a strong field ligand (large ),the complex ion is paramagnetic.
E)The geometric isomers of the complex ion have identical chemical properties.
A)The complex ion contains Co(I).
B)The complex ion exhibits cis and trans geometrical isomers,but no optical isomers.
C)The complex ion exhibits two geometrical isomers (cis and trans)and two optical isomers.
D)Since en is a strong field ligand (large ),the complex ion is paramagnetic.
E)The geometric isomers of the complex ion have identical chemical properties.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is true about coordination complexes?
A)The metal is a Lewis base and the ligands are Lewis acids.
B)Only complexes with coordination number six are found in nature.
C)When the ligands approach a transition metal ion in an octahedral field,the dxz,dyz,and dxy atomic orbitals are affected the least by the ligands.
D)None of the above is true.
E)All of the above are true.
A)The metal is a Lewis base and the ligands are Lewis acids.
B)Only complexes with coordination number six are found in nature.
C)When the ligands approach a transition metal ion in an octahedral field,the dxz,dyz,and dxy atomic orbitals are affected the least by the ligands.
D)None of the above is true.
E)All of the above are true.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
73
Calculate the total number of unpaired electrons in the following complex ions: Zn(OH2)62+,Ni(CN)42- (square planar),Co(NH3)63+ (strong field).
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
74
For the process Co(NH3)5Cl2+ + Cl- Co(NH3)4Cl2+ + NH3 what would be the ratio of cis to trans isomer in the product?
A)1 : 1
B)1 : 2
C)1 : 4
D)4 : 1
E)2 : 1
A)1 : 1
B)1 : 2
C)1 : 4
D)4 : 1
E)2 : 1

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
75
CuCl2- (linear)
A)0
B)1
C)2
D)4
E)5
A)0
B)1
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is paramagnetic?
A)Zn(H2O)62+
B)Co(NH3)63+ (strong field)
C)Cu(CN)32-
D)Mn(CN)62- (strong field)
E)none of these
A)Zn(H2O)62+
B)Co(NH3)63+ (strong field)
C)Cu(CN)32-
D)Mn(CN)62- (strong field)
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
77
Give the number of geometrical isomers for the octahedral compound [MA2B2C2],where A,B,and C represent ligands.
A)1
B)2
C)3
D)5
E)none of these
A)1
B)2
C)3
D)5
E)none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following are structural isomers?
I.coordination isomers
II.linkage isomers
III.geometrical isomers
IV.optical isomers
A)I,III
B)II,IV
C)I,III,IV
D)II,III
E)I,II
I.coordination isomers
II.linkage isomers
III.geometrical isomers
IV.optical isomers
A)I,III
B)II,IV
C)I,III,IV
D)II,III
E)I,II

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
79
Ni(NH3) 62+
A)0
B)1
C)2
D)4
E)5
A)0
B)1
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following ligands might give linkage isomers?
A)NO2-
B)SCN-
C)H2NHC2CH2NH2
D)A and B
E)A,B,and C
A)NO2-
B)SCN-
C)H2NHC2CH2NH2
D)A and B
E)A,B,and C

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck


