Exam 21: Transition Metals and Coordination Chemistry
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
The color of a transition metal complex results from:
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
C
How many unpaired electrons are found in each of the following complex ions?
-[Ni(CN)6]4-
Free
(Short Answer)
4.9/5  (48)
(48)
Correct Answer:
2
Fluoride ion ranks low in the spectrochemical series and produces a weak crystal field in complex ions.Based on this information,predict the number of unpaired electrons in CoF64-.
(Multiple Choice)
4.8/5  (38)
(38)
The transition metal __________ assists insulin in the control of blood sugar and may also be involved in the control of cholesterol.
(Multiple Choice)
4.7/5  (45)
(45)
Name the careful heat treatment of metals that provides the proper combination of strength without too much brittleness.
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following complexes would be diamagnetic (all electrons paired)? Assume the strong-field case for all complexes.
(Multiple Choice)
4.9/5  (24)
(24)
A complex ion is a charged species consisting of a metal ion surrounded by
(Multiple Choice)
4.8/5  (37)
(37)
Why do transition metals show a lot of chemical similarities within a given period?
(Multiple Choice)
4.7/5  (34)
(34)
Here are some crystal field representations of d electrons in an octahedral complex:
I) 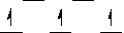 II)
II) 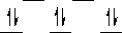 III)
III) 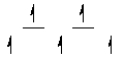 IV)
IV) 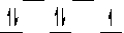 V)
V) 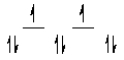 Choose the representation that fits the transition metal atom in the following species:
-K4Fe(CN)6
Choose the representation that fits the transition metal atom in the following species:
-K4Fe(CN)6
(Multiple Choice)
4.9/5  (40)
(40)
How many unpaired electrons are there in a complex ion having a d5 electron configuration and an octahedral geometry in the weak field case?
(Multiple Choice)
4.8/5  (36)
(36)
Which model(s)accounts for the magnetism and color of coordination compounds?
I.Lewis model
II.localized electron model
III.crystal field model
(Multiple Choice)
4.7/5  (31)
(31)
True or false: Transition metals show great similarities both within a given period and within a given vertical group.
(True/False)
4.7/5  (37)
(37)
How many unpaired electrons are found in each of the following complex ions?
-[Co(NH3) 4]2+
(Short Answer)
4.9/5  (41)
(41)
Which of the following statements concerning the complex ion Co(en)2Cl2+ is true? (en = ethylenediamine,NH2CH2CH2NH2)?
(Multiple Choice)
4.8/5  (35)
(35)
When the body adapts to high altitudes,it makes more __________ to adapt to lower oxygen concentrations in the blood.
(Multiple Choice)
4.8/5  (37)
(37)
A metal ion in a high-spin octahedral complex has two more unpaired electrons than the same ion does in a low-spin octahedral complex.The metal ion could be:
(Multiple Choice)
4.8/5  (37)
(37)
Showing 1 - 20 of 142
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)