Deck 16: Solubility and Complex Ion Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 16: Solubility and Complex Ion Equilibria
1
The solubility of an unknown salt,MZ2, at 25°C is 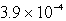 mol/L.What is the Ksp for MZ2 at 25°C?
mol/L.What is the Ksp for MZ2 at 25°C?
A)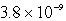
B)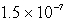
C)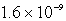
D)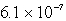
E)none of these
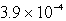 mol/L.What is the Ksp for MZ2 at 25°C?
mol/L.What is the Ksp for MZ2 at 25°C?A)
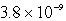
B)
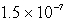
C)
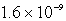
D)
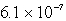
E)none of these
none of these
2
Calculate the concentration of chromate ion,CrO42-,in a saturated solution of CaCrO4 (Ksp = 7.08 10-4).
A)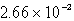 M
M
B)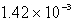 M
M
C)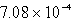 M
M
D)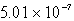 M
M
E)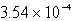 M
M
A)
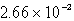 M
MB)
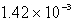 M
MC)
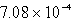 M
MD)
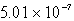 M
ME)
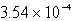 M
M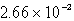 M
M 3
The solubility of silver phosphate,Ag3PO4, at 25°C is 1.60 10-5 mol/L.What is the Ksp for the silver phosphate at 25°C?
A)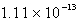
B)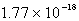
C)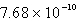
D)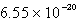
E)none of these
A)
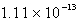
B)
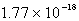
C)
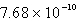
D)
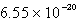
E)none of these
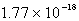
4
Silver chromate,Ag2CrO4,has a Ksp of 8.96 10-12.Calculate the solubility in mol/L of silver chromate.
A)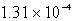 M
M
B)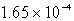 M
M
C)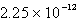 M
M
D)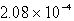 M
M
E)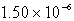 M
M
A)
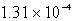 M
MB)
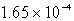 M
MC)
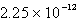 M
MD)
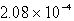 M
ME)
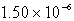 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
The solubility of Cd(OH)2 in water is 1.67 10-5 mol/L.The Ksp value for Cd(OH)2 is
A)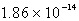
B)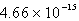
C)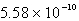
D)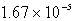
E)none of these
A)
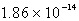
B)
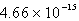
C)
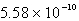
D)
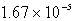
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
The molar solubility of PbI2 is 1.52 10-3 M.Calculate the value of Ksp for PbI2.
A)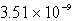
B)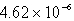
C)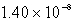
D)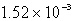
E)none of these
A)
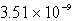
B)
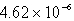
C)
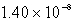
D)
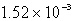
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
The 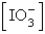 in a saturated solution of
in a saturated solution of 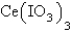 is 5.93 10-3 M.Calculate the Ksp for
is 5.93 10-3 M.Calculate the Ksp for 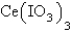 .
.
A)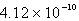
B)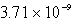
C)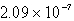
D)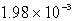
E)none of these
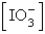 in a saturated solution of
in a saturated solution of 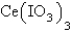 is 5.93 10-3 M.Calculate the Ksp for
is 5.93 10-3 M.Calculate the Ksp for 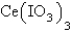 .
.A)
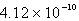
B)
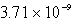
C)
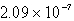
D)
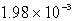
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The correct mathematical expression for finding the molar solubility (s)of Sn(OH)2 is:
A)2s2 = Ksp
B)2s3 = Ksp
C)108s5 = Ksp
D)4s3 = Ksp
E)8s3 = Ksp
A)2s2 = Ksp
B)2s3 = Ksp
C)108s5 = Ksp
D)4s3 = Ksp
E)8s3 = Ksp

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
A 300.0-mL saturated solution of copper(II)peroidate,Cu(IO4)2,contains 0.30 grams of dissolved salt.Determine the Ksp.
A)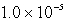
B)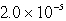
C)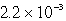
D)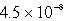
E)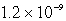
A)
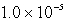
B)
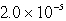
C)
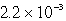
D)
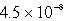
E)
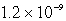

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
An unknown salt,M2Z,has a Ksp of 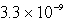 .Calculate the solubility in mol/L of M2Z.
.Calculate the solubility in mol/L of M2Z.
A)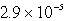 M
M
B)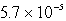 M
M
C)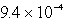 M
M
D)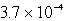 M
M
E)none of the above
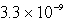 .Calculate the solubility in mol/L of M2Z.
.Calculate the solubility in mol/L of M2Z.A)
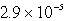 M
MB)
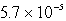 M
MC)
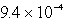 M
MD)
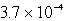 M
ME)none of the above

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the concentration of the silver ion in a saturated solution of silver chloride,AgCl (Ksp = 1.57 10-10).
A)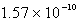
B)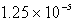
C)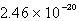
D)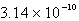
E)none of these
A)
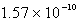
B)
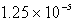
C)
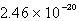
D)
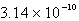
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
The solubility of an unknown salt,M3Z2, at 25°C is 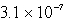 mol/L.What is the Ksp for M3Z2 at 25°C?
mol/L.What is the Ksp for M3Z2 at 25°C?
A)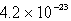
B)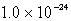
C)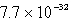
D)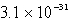
E)none of these
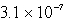 mol/L.What is the Ksp for M3Z2 at 25°C?
mol/L.What is the Ksp for M3Z2 at 25°C?A)
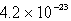
B)
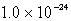
C)
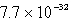
D)
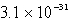
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
The solubility of an unknown salt,MZ3, at 25°C is 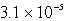 mol/L.What is the Ksp for MZ3 at 25°C?
mol/L.What is the Ksp for MZ3 at 25°C?
A)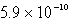
B)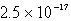
C)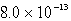
D)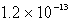
E)none of these
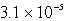 mol/L.What is the Ksp for MZ3 at 25°C?
mol/L.What is the Ksp for MZ3 at 25°C?A)
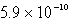
B)
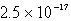
C)
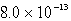
D)
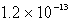
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
The solubility in mol/L of Ag2CrO4 is 1.8 10-4 M.Calculate the Ksp for this compound.
A)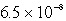
B)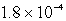
C)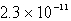
D)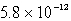
E)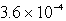
A)
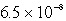
B)
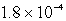
C)
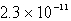
D)
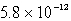
E)
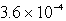

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
It is observed that 7.50 mmol of BaF2 will dissolve in 1.0 L of water.Use these data to calculate the value of Ksp for barium fluoride.
A)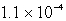
B)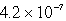
C)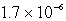
D)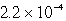
E)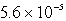
A)
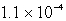
B)
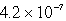
C)
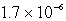
D)
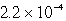
E)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
The solubility of CaSO4 in pure water at 0oC is 1.14 gram(s)per liter.The value of the solubility product is
A)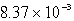
B)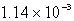
C)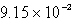
D)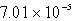
E)none of these
A)
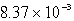
B)
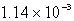
C)
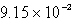
D)
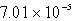
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
An unknown salt,M3Z,has a Ksp of 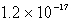 .Calculate the solubility in mol/L of M3Z.
.Calculate the solubility in mol/L of M3Z.
A)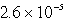 M
M
B)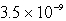 M
M
C)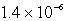 M
M
D)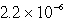 M
M
E)none of the above
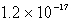 .Calculate the solubility in mol/L of M3Z.
.Calculate the solubility in mol/L of M3Z.A)
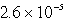 M
MB)
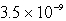 M
MC)
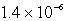 M
MD)
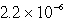 M
ME)none of the above

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
The concentration of OH- in a saturated solution of Mg(OH)2 is 3.63 10-4 M.The Ksp of Mg(OH)2 is
A)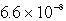
B)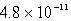
C)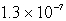
D)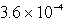
E)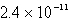
A)
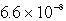
B)
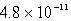
C)
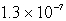
D)
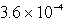
E)
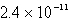

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
Barium carbonate has a measured solubility of 4.03 10-5 at 25°C.Determine the Ksp.
A)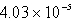
B)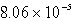
C)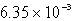
D)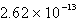
E)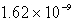
A)
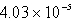
B)
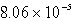
C)
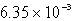
D)
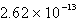
E)
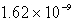

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Find the solubility (in mol/L)of lead(II)chloride,PbCl2,at 25°C.Ksp = 1.59 10-5.
A)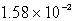
B)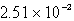
C)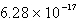
D)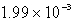
E)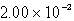
A)
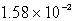
B)
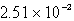
C)
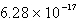
D)
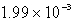
E)
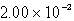

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the solubility of Ag2CrO4 (Ksp = 9.0 10-12)in a 0.049 M AgNO3 solution.
A)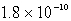 mol/L
mol/L
B)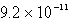 mol/L
mol/L
C)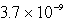 mol/L
mol/L
D)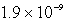 mol/L
mol/L
E)none of these
A)
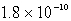 mol/L
mol/LB)
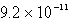 mol/L
mol/LC)
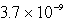 mol/L
mol/LD)
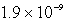 mol/L
mol/LE)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
Chromate ion is added to a saturated solution of Ag2CrO4 to reach 0.78 M CrO42-.Calculate the final concentration of silver ion at equilibrium (Ksp for Ag2CrO4 is 9.0 10-12).
A)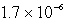 M
M
B)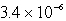 M
M
C)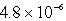 M
M
D)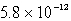 M
M
E)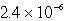 M
M
A)
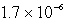 M
MB)
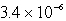 M
MC)
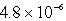 M
MD)
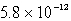 M
ME)
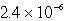 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
The solubility of La(IO3)3 in a 0.42 M KIO3 solution is 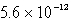 mol/L.Calculate the Ksp for La(IO3)3.
mol/L.Calculate the Ksp for La(IO3)3.
A)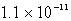
B)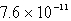
C)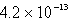
D)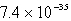
E)none of these
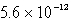 mol/L.Calculate the Ksp for La(IO3)3.
mol/L.Calculate the Ksp for La(IO3)3.A)
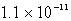
B)
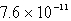
C)
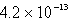
D)
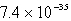
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
Solubility Products (Ksp) 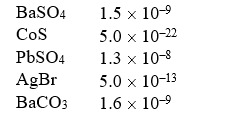
Which of the following compounds is the most soluble (in moles/liter)?
A)BaSO4
B)CoS
C)PbSO4
D)AgBr
E)BaCO3
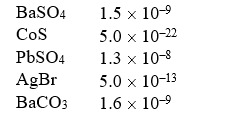
Which of the following compounds is the most soluble (in moles/liter)?
A)BaSO4
B)CoS
C)PbSO4
D)AgBr
E)BaCO3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following salts shows the lowest solubility in water? (Ksp values: Ag2S = 1.6 10-49;Bi2S3 = 1.0 10-72;HgS = 1.6 10-54;Mg(OH)2 = 8.9 10-12;MnS = 2.3 10-13)
A)Bi2S3
B)Ag2S
C)MnS
D)HgS
E)Mg(OH)2
A)Bi2S3
B)Ag2S
C)MnS
D)HgS
E)Mg(OH)2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
The Ksp of an unknown salt,MZ2,is 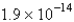 .Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.
.Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.
A)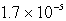 M
M
B)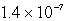 M
M
C)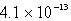 M
M
D)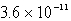 M
M
E)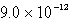 M
M
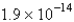 .Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.
.Calculate the solubility (in mol/L)of MZ2 in a 0.0230 M solution of CaZ2.A)
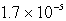 M
MB)
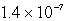 M
MC)
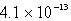 M
MD)
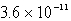 M
ME)
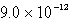 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds has the lowest solubility in mol/L in water at 25°C?
A)Ag3PO4 Ksp = 1.8 10-18
B)Sn(OH)2 Ksp = 3 10-27
C)CdS Ksp = 1.0 10-28
D)CaSO4 Ksp = 6.1 10-5
E)Al(OH)3 Ksp = 2 10-33
A)Ag3PO4 Ksp = 1.8 10-18
B)Sn(OH)2 Ksp = 3 10-27
C)CdS Ksp = 1.0 10-28
D)CaSO4 Ksp = 6.1 10-5
E)Al(OH)3 Ksp = 2 10-33

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
The solubility of an unknown salt,M2Z,in a 0.0768 M CaZ solution is 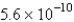 mol/L.Calculate the Ksp for M2Z.
mol/L.Calculate the Ksp for M2Z.
A)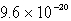
B)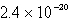
C)
D)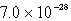
E)none of these
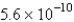 mol/L.Calculate the Ksp for M2Z.
mol/L.Calculate the Ksp for M2Z.A)
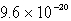
B)
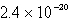
C)
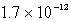
D)
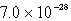
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
The solubility of silver phosphate,Ag3PO4,at 25°C is 1.55 10-5 mol/L.Determine the concentration of the Ag+ ion in a saturated solution.
A)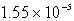 M
M
B)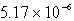 M
M
C)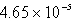 M
M
D)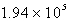 M
M
E)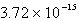 M
M
A)
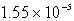 M
MB)
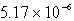 M
MC)
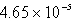 M
MD)
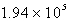 M
ME)
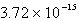 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the solubility of Ca3(PO4)2 (Ksp = 1.3 10-32)in a 0.048 M Ca(NO3)2 solution.
A)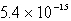 mol/L
mol/L
B)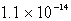 mol/L
mol/L
C)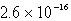 mol/L
mol/L
D)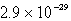 mol/L
mol/L
E)none of these
A)
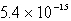 mol/L
mol/LB)
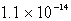 mol/L
mol/LC)
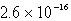 mol/L
mol/LD)
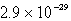 mol/L
mol/LE)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
The Ksp of PbSO4 is 1.3 10-8.Calculate the solubility (in mol/L)of PbSO4 in a 0.0037 M solution of Na2SO4.
A)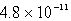 M
M
B)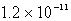 M
M
C)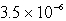 M
M
D)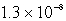 M
M
E)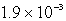 M
M
A)
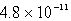 M
MB)
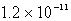 M
MC)
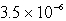 M
MD)
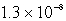 M
ME)
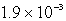 M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following compounds has the lowest solubility in mol/L in water?
A)Al(OH)3 Ksp = 2 10-32
B)CdS Ksp = 1.0 10-28
C)PbSO4 Ksp = 1.3 10-8
D)Sn(OH)2 Ksp = 3 10-27
E)MgC2O4 Ksp = 8.6 10-5
A)Al(OH)3 Ksp = 2 10-32
B)CdS Ksp = 1.0 10-28
C)PbSO4 Ksp = 1.3 10-8
D)Sn(OH)2 Ksp = 3 10-27
E)MgC2O4 Ksp = 8.6 10-5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
How many moles of Fe(OH)2 [Ksp = 1.8 10-15] will dissolve in 1.0 liter of water buffered at pH = 10.37?
A)![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_6247_93a6_6374d120731f_TB6423_11.jpg)
B)![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_8958_93a6_39ecb3db2875_TB6423_11.jpg)
C)![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_8959_93a6_7302bcc213e8_TB6423_11.jpg)
D)![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_895a_93a6_f3557aa0ffda_TB6423_11.jpg)
E)![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_895b_93a6_13ae23d487fb_TB6423_11.jpg)
A)
![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_6247_93a6_6374d120731f_TB6423_11.jpg)
B)
![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_8958_93a6_39ecb3db2875_TB6423_11.jpg)
C)
![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_8959_93a6_7302bcc213e8_TB6423_11.jpg)
D)
![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_895a_93a6_f3557aa0ffda_TB6423_11.jpg)
E)
![<strong>How many moles of Fe(OH)<sub>2</sub> [K<sub>sp</sub> = 1.8 \times 10<sup>-</sup><sup>15</sup>] will dissolve in 1.0 liter of water buffered at pH = 10.37?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6423/11eaa8f0_66e0_895b_93a6_13ae23d487fb_TB6423_11.jpg)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
The solubility of Mg(OH)2 (Ksp = 8.9 10-12)in 1.0 L of a solution buffered (with large capacity)at pH 9.73 is:
A)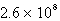 moles
moles
B)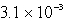 moles
moles
C)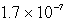 moles
moles
D)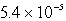 moles
moles
E)none of these
A)
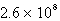 moles
molesB)
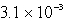 moles
molesC)
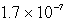 moles
molesD)
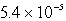 moles
molesE)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
The molar solubility of AgCl (Ksp = 1.6 10-10)in 0.0035 M sodium chloride at 25°C is:
A)0.0035
B)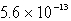
C)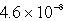
D)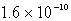
E)none of these
A)0.0035
B)
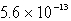
C)
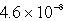
D)
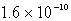
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
In a solution prepared by adding excess PbI2 (Ksp = 1.44 10-8)to water,the [I-] at equilibrium is:
A)![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0d_93a6_930a5183db52_TB6423_11.jpg) mol/L
mol/L
B)![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0e_93a6_e3c37ce2d5de_TB6423_11.jpg) mol/L
mol/L
C)![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0f_93a6_6dd153a3bbc6_TB6423_11.jpg) mol/L
mol/L
D)![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66e0_1420_93a6_79b0005ec6e9_TB6423_11.jpg) mol/L
mol/L
E)![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66e0_1421_93a6_dd44481f3ce4_TB6423_11.jpg) mol/L
mol/L
A)
![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0d_93a6_930a5183db52_TB6423_11.jpg) mol/L
mol/LB)
![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0e_93a6_e3c37ce2d5de_TB6423_11.jpg) mol/L
mol/LC)
![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66df_ed0f_93a6_6dd153a3bbc6_TB6423_11.jpg) mol/L
mol/LD)
![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66e0_1420_93a6_79b0005ec6e9_TB6423_11.jpg) mol/L
mol/LE)
![<strong>In a solution prepared by adding excess PbI<sub>2</sub> (K<sub>sp</sub> = 1.44 \times 10<sup>-</sup><sup>8</sup>)to water,the [I<sup>-</sup>] at equilibrium is:</strong> A) mol/L B) mol/L C) mol/L D) mol/L E) mol/L](https://storage.examlex.com/TB6423/11eaa8f0_66e0_1421_93a6_dd44481f3ce4_TB6423_11.jpg) mol/L
mol/L
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
The Ksp for PbF2 is 4.0 10-8.If a 0.040 M NaF solution is saturated with PbF2,what is the [Pb2+] in the solution?
A)![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e0_fe98_93a6_f99873761bf1_TB6423_11.jpg) M
M
B)![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25a9_93a6_bd694eb91b62_TB6423_11.jpg) M
M
C)![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25aa_93a6_05ee747f5232_TB6423_11.jpg) M
M
D)![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25ab_93a6_331e5211e4e1_TB6423_11.jpg) M
M
E)![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25ac_93a6_0d85e07d06b4_TB6423_11.jpg) M
M
A)
![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e0_fe98_93a6_f99873761bf1_TB6423_11.jpg) M
MB)
![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25a9_93a6_bd694eb91b62_TB6423_11.jpg) M
MC)
![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25aa_93a6_05ee747f5232_TB6423_11.jpg) M
MD)
![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25ab_93a6_331e5211e4e1_TB6423_11.jpg) M
ME)
![<strong>The K<sub>sp</sub> for PbF<sub>2</sub> is 4.0 \times 10<sup>-</sup><sup>8</sup>.If a 0.040 M NaF solution is saturated with PbF<sub>2</sub>,what is the [Pb<sup>2+</sup>] in the solution?</strong> A) M B) M C) M D) M E) M](https://storage.examlex.com/TB6423/11eaa8f0_66e1_25ac_93a6_0d85e07d06b4_TB6423_11.jpg) M
M
Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the concentration of Al3+ in a saturated aqueous solution of Al(OH)3 (Ksp = 2.2 10-32).
A)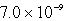
B)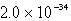
C)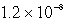
D)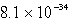
E)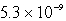
A)
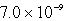
B)
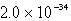
C)
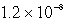
D)
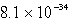
E)
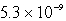

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
The molar solubility of BaCO3 (Ksp = 1.6 10-9)in 0.10 M BaCl2 solution is:
A)1.6 10-10
B)4.0 10-5
C)7.4 10-4
D)0.10
E)none of these
A)1.6 10-10
B)4.0 10-5
C)7.4 10-4
D)0.10
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
The Ksp of AgI is 1.5 10-16.Calculate the solubility in mol/L of AgI in a 0.30 M NaI solution.
A)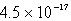
B)0.30
C)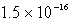
D)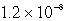
E)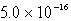
A)
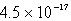
B)0.30
C)
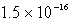
D)
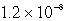
E)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles of CaF2 will dissolve in 3.0 liters of 0.089 M NaF solution? (Ksp for CaF2 = 4.0 10-11)
A)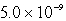
B)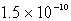
C)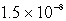
D)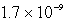
E)none of these
A)
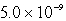
B)
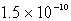
C)
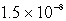
D)
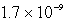
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following solid salts is more soluble in 1.0 M H+ than in pure water?
A)NaCl
B)KCl
C)FePO4
D)AgCl
E)KNO3
A)NaCl
B)KCl
C)FePO4
D)AgCl
E)KNO3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
What is the limiting reagent in the formation of the lead chloride?
A)Pb2+
B)Cl-
C)(NO3)-
D)PbCl2
E)Pb(NO3)2
A)Pb2+
B)Cl-
C)(NO3)-
D)PbCl2
E)Pb(NO3)2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
What is the best way to ensure complete precipitation of SnS from a saturated H2S solution?
A)Add more H2S.
B)Add a strong acid.
C)Add a weak acid.
D)Add a strong base.
E)Add a weak base.
A)Add more H2S.
B)Add a strong acid.
C)Add a weak acid.
D)Add a strong base.
E)Add a weak base.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following solid salts is more soluble in 1.0 M H+ than in pure water?
A)NaCl
B)CaCO3
C)KCl
D)AgCl
E)KNO3
A)NaCl
B)CaCO3
C)KCl
D)AgCl
E)KNO3

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
You have a solution consisting of 0.10 M Cl- and 0.10 M CrO42-.You add 0.10 M silver nitrate dropwise to this solution.Given that the Ksp for Ag2CrO4 is 9.0 10-12,and that for AgCl is 1.6 10-10,which of the following will precipitate first?
A)silver chloride
B)silver chromate
C)silver nitrate
D)cannot be determined by the information given
E)none of these
A)silver chloride
B)silver chromate
C)silver nitrate
D)cannot be determined by the information given
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
You have two salts,AgX and AgY,with very similar Ksp values.You know that Ka for HX is much greater than Ka for HY.Which salt is more soluble in acidic solution?
A)AgX
B)AgY
C)They are equally soluble in acidic solution.
D)Cannot be determined by the information given.
E)None of these (A-D).
A)AgX
B)AgY
C)They are equally soluble in acidic solution.
D)Cannot be determined by the information given.
E)None of these (A-D).

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
The two salts AgX and AgY exhibit very similar solubilities in water.It is known that the salt AgX is much more soluble in acid than is AgY.What can be said about the relative strengths of the acids HX and HY?
A)Nothing.
B)HY is stronger than HX.
C)HX is stronger than HY.
D)The acids are weak acids and have equal values for Ka.
E)Both acids are strong.
A)Nothing.
B)HY is stronger than HX.
C)HX is stronger than HY.
D)The acids are weak acids and have equal values for Ka.
E)Both acids are strong.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of Ca(NO3)2 must be added to 1.0 L of a 0.182 M KF solution to begin precipitation of CaF2? For CaF2,Ksp = 4.0 10-11.
A)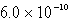
B)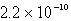
C)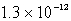
D)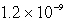
E)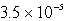
A)
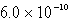
B)
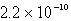
C)
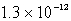
D)
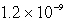
E)
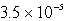

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
Will precipitation occur?
A)Yes.
B)No.
C)Maybe,it depends on the temperature.
D)Maybe,it depends on the limiting reagent concentration.
E)None of these.
A)Yes.
B)No.
C)Maybe,it depends on the temperature.
D)Maybe,it depends on the limiting reagent concentration.
E)None of these.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the solubility of Cu(OH)2 in a solution buffered at pH = 7.59.(Ksp = 1.6 10-19)
A)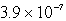 M
M
B)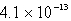 M
M
C)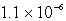 M
M
D)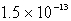 M
M
E)none of these
A)
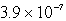 M
MB)
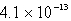 M
MC)
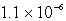 M
MD)
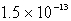 M
ME)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
The Ksp of Al(OH)3 is 2 10-32.At what pH will a 0.5 M Al3+ solution begin to show precipitation of Al(OH)3?
A)3.5
B)10.5
C)1.0
D)6.0
E)3.1
A)3.5
B)10.5
C)1.0
D)6.0
E)3.1

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
The solubility of AgCl in water is _____ the solubility of AgCl in strong acid at the same temperature.
A)greater than
B)less than
C)about the same as
D)cannot be determined
E)much different from
A)greater than
B)less than
C)about the same as
D)cannot be determined
E)much different from

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
Given the following Ksp values,which statement about solubility in mol/L in water is correct?
![<strong>Given the following K<sub>sp</sub> values,which statement about solubility in mol/L in water is correct? </strong> A)PbCrO<sub>4</sub>,Zn(OH)<sub>2</sub>,and Pb(OH)<sub>2</sub> have equal solubilities in water. B)PbCrO<sub>4</sub> has the lowest solubility in water. C)The solubility of MnS in water will not be pH dependent. D)MnS has the highest molar solubility in water. E)A saturated PbCrO<sub>4</sub> solution will have a higher [Pb<sup>2+</sup>] than a saturated Pb(OH)<sub>2</sub> solution.](https://storage.examlex.com/TB6423/11efdc24_1e44_7205_8f65_f347c5c118ae_TB6423_00.jpg)
A)PbCrO4,Zn(OH)2,and Pb(OH)2 have equal solubilities in water.
B)PbCrO4 has the lowest solubility in water.
C)The solubility of MnS in water will not be pH dependent.
D)MnS has the highest molar solubility in water.
E)A saturated PbCrO4 solution will have a higher [Pb2+] than a saturated Pb(OH)2 solution.
![<strong>Given the following K<sub>sp</sub> values,which statement about solubility in mol/L in water is correct? </strong> A)PbCrO<sub>4</sub>,Zn(OH)<sub>2</sub>,and Pb(OH)<sub>2</sub> have equal solubilities in water. B)PbCrO<sub>4</sub> has the lowest solubility in water. C)The solubility of MnS in water will not be pH dependent. D)MnS has the highest molar solubility in water. E)A saturated PbCrO<sub>4</sub> solution will have a higher [Pb<sup>2+</sup>] than a saturated Pb(OH)<sub>2</sub> solution.](https://storage.examlex.com/TB6423/11efdc24_1e44_7205_8f65_f347c5c118ae_TB6423_00.jpg)
A)PbCrO4,Zn(OH)2,and Pb(OH)2 have equal solubilities in water.
B)PbCrO4 has the lowest solubility in water.
C)The solubility of MnS in water will not be pH dependent.
D)MnS has the highest molar solubility in water.
E)A saturated PbCrO4 solution will have a higher [Pb2+] than a saturated Pb(OH)2 solution.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
The Ksp for BaF2 is 2.4 10-5.When 10 mL of 0.01 M NaF is mixed with 10 mL of 0.01 M BaNO3,will a precipitate form?
A)No,because Q is 1 10-12 and since it is less than Ksp no precipitate will form.
B)Yes,because Q is 1 10-12 and since it is less than Ksp a precipitate will form.
C)No,because Q is 1.25 10-7 and since it is less than Ksp no precipitate will form.
D)Yes,because Q is 1.25 10-7 and since it is less than Ksp a precipitate will form.
E)None of the above.
A)No,because Q is 1 10-12 and since it is less than Ksp no precipitate will form.
B)Yes,because Q is 1 10-12 and since it is less than Ksp a precipitate will form.
C)No,because Q is 1.25 10-7 and since it is less than Ksp no precipitate will form.
D)Yes,because Q is 1.25 10-7 and since it is less than Ksp a precipitate will form.
E)None of the above.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
A 50.0-mL sample of 0.100 M Ca(NO3)2 is mixed with 50.00 mL of 0.200 M NaF.When the system has come to equilibrium,which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 10-11. Moles Solid CaF2
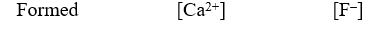
A)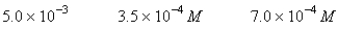
B)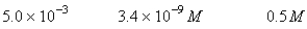
C)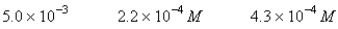
D)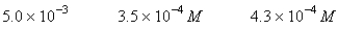
E)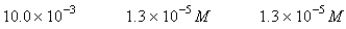
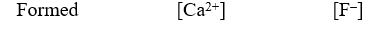
A)
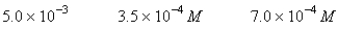
B)
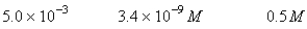
C)
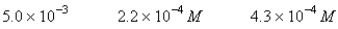
D)
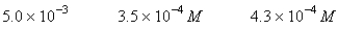
E)
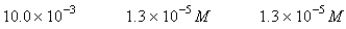

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
Determine the equilibrium concentration of the chloride ion.
A)3.9 10-4
B)8.0 10-6
C)2.8 10-3
D)6.1 10-2
E)none of these
A)3.9 10-4
B)8.0 10-6
C)2.8 10-3
D)6.1 10-2
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
The following questions refer to the following system: 3.5 102 mL of 3.2 M Pb(NO3)2 and 2.0 102 mL of 0.020 M NaCl are added together.Ksp for the lead chloride is 1.6 10-5.
-Determine the ion product.
A)1.1 10-4
B)1.5 10-2
C)7.8 10-3
D)8.1 10-4
E)none of these
-Determine the ion product.
A)1.1 10-4
B)1.5 10-2
C)7.8 10-3
D)8.1 10-4
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
The solubility in mol/L of M(OH)2 in 0.053 M KOH is 1.0 10-5 mol/L.What is the Ksp for M(OH)2?
A)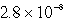
B)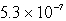
C)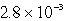
D)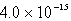
E)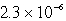
A)
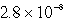
B)
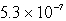
C)
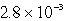
D)
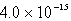
E)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
The best explanation for the dissolution of ZnS in dilute HCl is that:
A)The zinc ion is amphoteric.
B)The sulfide-ion concentration is decreased by the formation of H2S.
C)the sulfide-ion concentration is decreased by oxidation to sulfur.
D)the zinc-ion concentration is decreased by the formation of a chloro complex.
E)The solubility product of ZnCl2 is less than that of ZnS.
A)The zinc ion is amphoteric.
B)The sulfide-ion concentration is decreased by the formation of H2S.
C)the sulfide-ion concentration is decreased by oxidation to sulfur.
D)the zinc-ion concentration is decreased by the formation of a chloro complex.
E)The solubility product of ZnCl2 is less than that of ZnS.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
A solution is 0.010 M in each of Pb(NO3)2,Mn(NO3)2,and Zn(NO3)2.Solid NaOH is added until the pH of the solution is 8.50.Which of the following statements is true?

A)No precipitate will form.
B)Only Pb(OH)2 will precipitate.
C)Only Mn(OH)2 will precipitate.
D)Only Zn(OH)2 and Pb(OH)2 will precipitate.
E)All three hydroxides will precipitate.

A)No precipitate will form.
B)Only Pb(OH)2 will precipitate.
C)Only Mn(OH)2 will precipitate.
D)Only Zn(OH)2 and Pb(OH)2 will precipitate.
E)All three hydroxides will precipitate.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following solid salts should be more soluble in 1.0 M NH3 than in water?
A)Na2CO3
B)KCl
C)AgBr
D)KNO3
E)none of these
A)Na2CO3
B)KCl
C)AgBr
D)KNO3
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
The overall Kf for the complex ion Ag(NH3)2+ is 1.7 107.The Ksp for AgI is 1.5 10-16.What is the molar solubility of AgI in a solution that is 2.0 M in NH3?
A)1.5 10-9
B)1.3 10-3
C)1.0 10-4
D)5.8 10-12
E)8.4 10-5
A)1.5 10-9
B)1.3 10-3
C)1.0 10-4
D)5.8 10-12
E)8.4 10-5

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
In the qualitative analysis scheme for metal ions,how are the Analytical Group II cations separated from the cations of Analytical Groups III-V?
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
In the qualitative analysis scheme for metal ions,how are the Analytical Group III cations separated from the cations of Analytical Groups IV and V?
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
The Ksp for Mn(OH)2 is 2.0 10-13.At what pH will Mn(OH)2 begin to precipitate from a solution in which the initial concentration of Mn2+ is 0.10 M?
A)6.47
B)13.3
C)5.85
D)7.03
E)8.15
A)6.47
B)13.3
C)5.85
D)7.03
E)8.15

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
The concentration of Mg2+ in seawater is 0.052 M.At what pH will 99% of the Mg2+ be precipitated as the hydroxide? [Ksp for Mg(OH)2 = 8.9 10-12]
A)8.35
B)9.22
C)6.50
D)10.12
E)4.86
A)8.35
B)9.22
C)6.50
D)10.12
E)4.86

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
An industrial plant processes its waste water through a sedimentation tank that removes hazardous metals by precipitating them as insoluble carbonate salts.If sodium carbonate is gradually added to the tank,what would be the order of precipitation of the metals,Pb2+,Cu2+,Hg22+,and Zn2+ if each is 1.0 10-4 M? ( Ksp PbCO3 = 7.4 10-14,Ksp CuCO3 = 1.4 10-10,Ksp Hg2CO3 = 8.9 10-17,and Ksp ZnCO3 = 1.4 10-11)
A)Pb2+,Hg22+,Zn2+,Cu2+
B)Hg22+,Pb2+,Zn2+,Cu2+
C)Cu2+,Zn2+,Pb2+,Hg22+
D)Cu2+,Zn2+,Hg22+,Pb2+
E)All metal ions will precipitate at the same time.
A)Pb2+,Hg22+,Zn2+,Cu2+
B)Hg22+,Pb2+,Zn2+,Cu2+
C)Cu2+,Zn2+,Pb2+,Hg22+
D)Cu2+,Zn2+,Hg22+,Pb2+
E)All metal ions will precipitate at the same time.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
The Kf for the complex ion Ag(NH3)2+ is 1.7 107.The Ksp for AgCl is 1.6 10-10.Calculate the molar solubility of AgCl in 1.0 M NH3.
A)5.2 10-2
B)4.7 10-2
C)2.9 10-3
D)1.3 10-5
E)1.7 10-10
A)5.2 10-2
B)4.7 10-2
C)2.9 10-3
D)1.3 10-5
E)1.7 10-10

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
When a mixture containing cations of Analytical Groups I-III is treated with H2S in acidic solution,which cations are expected to precipitate?
A)Analytical Group I only
B)Analytical Group II only
C)Analytical Group III only
D)Analytical Groups I and II
E)Analytical Groups II and III
A)Analytical Group I only
B)Analytical Group II only
C)Analytical Group III only
D)Analytical Groups I and II
E)Analytical Groups II and III

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
A 0.012-mol sample of Na2SO4 is added to 400 mL of each of two solutions.One solution contains 1.5 10-3 M BaCl2;the other contains 1.5 10-3 M CaCl2.Given that Ksp for BaSO4 = 1.5 10-9 and Ksp for CaSO4 = 6.1 10-5:
A)BaSO4 would precipitate but CaSO4 would not.
B)CaSO4 would precipitate but BaSO4 would not.
C)Both BaSO4 and CaSO4 would precipitate.
D)Neither BaSO4 nor CaSO4 would precipitate.
E)Not enough information is given to determine if precipitation would occur.
A)BaSO4 would precipitate but CaSO4 would not.
B)CaSO4 would precipitate but BaSO4 would not.
C)Both BaSO4 and CaSO4 would precipitate.
D)Neither BaSO4 nor CaSO4 would precipitate.
E)Not enough information is given to determine if precipitation would occur.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Consider a solution containing the following cations: Na+,Hg2+,Mn2+,Al3+ and Ag+.Treatment of the solution with dilute HCl followed by saturation with H2S results in formation of precipitate(s).Which ions still remain in solution (i.e. ,did not precipitate)?
A)Ag+ only
B)Na+,Hg2+,Al3+
C)Ag+ and Hg2+
D)Na+,Al3+,and Mn2+
E)Na+ only
A)Ag+ only
B)Na+,Hg2+,Al3+
C)Ag+ and Hg2+
D)Na+,Al3+,and Mn2+
E)Na+ only

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
In the classic scheme for qualitative analysis,the cations of Analytical Group IV are precipitated as phosphates or carbonates.Analytical Group IV consists of
A)alkali metals
B)alkaline earth elements
C)the halogens
D)transition metals having +2 ions
E)none of these
A)alkali metals
B)alkaline earth elements
C)the halogens
D)transition metals having +2 ions
E)none of these

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
Sodium chloride is added slowly to a solution that is 0.010 M in Cu+,Ag+,and Au+.The Ksp values for the chloride salts are 1.9 10-7,1.6 10-10,and 2.0 10-13,respectively.Which compound will precipitate first?
A)CuCl
B)AgCl
C)AuCl
D)All will precipitate at the same time.
E)Cannot be determined.
A)CuCl
B)AgCl
C)AuCl
D)All will precipitate at the same time.
E)Cannot be determined.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
What is the maximum concentration of iodide ions that will precipitate AgI but not PbI2 from a solution that is 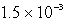 M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
A)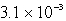 M
M
B)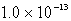 M
M
C)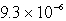 M
M
D)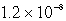 M
M
E)None of these;PbI2 will always precipitate before AgI.
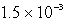 M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.A)
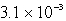 M
MB)
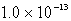 M
MC)
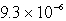 M
MD)
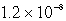 M
ME)None of these;PbI2 will always precipitate before AgI.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
What is the maximum concentration of carbonate ions that will precipitate BaCO3 but not MgCO3 from a solution that is 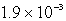 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
A)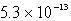 M
M
B)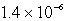 M
M
C)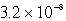 M
M
D)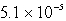 M
M
E)None of these;MgCO3 will always precipitate before BaCO3.
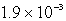 M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.
M each in Mg2+ and Ba2+? For MgCO3,Ksp = 1.0 10-15 and for BaCO3,Ksp = 2.6 10-9.A)
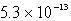 M
MB)
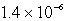 M
MC)
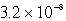 M
MD)
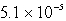 M
ME)None of these;MgCO3 will always precipitate before BaCO3.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
If 30 mL of 5.0 10-4 M Ca(NO3)2 are added to 70 mL of 2.0 10-4 M NaF,will a precipitate occur? (Ksp of CaF2 = 4.0 10-11)
A)No,because the ion product is greater than Ksp.
B)Yes,because the ion product is less than Ksp.
C)No,because the ion product is less than Ksp.
D)Not enough information is given.
E)Yes,because the ion product is greater than Ksp.
A)No,because the ion product is greater than Ksp.
B)Yes,because the ion product is less than Ksp.
C)No,because the ion product is less than Ksp.
D)Not enough information is given.
E)Yes,because the ion product is greater than Ksp.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
A 100.-mL sample of solution contains 10.0 mmol of Ca2+ ion.How many mmol of solid Na2SO4 must be added in order to cause precipitation of 99.9% of the calcium as CaSO4? The Ksp of CaSO4 is 6.1 10-5.Assume the volume remains constant.
A)17.4
B)10.0
C)61.0
D)71.0
E)6.1
A)17.4
B)10.0
C)61.0
D)71.0
E)6.1

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
In the qualitative analysis scheme for metal ions,how are the Analytical Group I cations separated from the other cations?
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates
A)by addition of HCl,forming insoluble metal chlorides
B)by addition of H2SO4,forming insoluble metal sulfates
C)by addition of H2S in acidic solution,forming insoluble metal sulfides
D)by addition of H2S in basic solution,forming insoluble metal sulfides or hydroxides
E)by addition of (NH4)2CO3 or (NH4)3PO4,forming insoluble metal carbonates or phosphates

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
A solution contains 0.018 moles each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s)precipitates out?
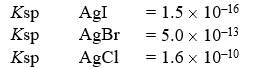
A)0.0 g
B)1.7 g
C)2.6 g
D)3.3 g
E)5.0 g
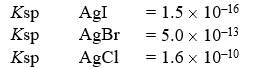
A)0.0 g
B)1.7 g
C)2.6 g
D)3.3 g
E)5.0 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck


