Exam 16: Solubility and Complex Ion Equilibria
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
The molar solubility of PbI2 is 1.52 10-3 M.Calculate the value of Ksp for PbI2.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
Given the following Ksp values,which statement about solubility in mol/L in water is correct?
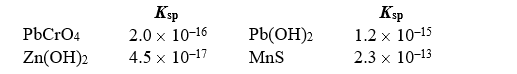
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
In the qualitative analysis scheme for metal ions,how are the Analytical Group I cations separated from the other cations?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
The best explanation for the dissolution of ZnS in dilute HCl is that:
(Multiple Choice)
4.9/5  (31)
(31)
The Ksp for Mn(OH)2 is 2.0 10-13.At what pH will Mn(OH)2 begin to precipitate from a solution in which the initial concentration of Mn2+ is 0.10 M?
(Multiple Choice)
4.9/5  (32)
(32)
The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO3)2 are mixed with 1.0 L of 1.0 M Na2C2O4.The oxalate ion,C2O4,acts as a ligand to form a complex ion with the Mn2+ ion with a coordination number of two.
Mn2+ + C2O42-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -What is the equilibrium constant for the following formation? Mn<sup>2+</sup> + 2C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d99_93a6_21fcac416c0b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
MnC2O4
K1 = 7.9 103
[Mn(C2O4)2]2-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -What is the equilibrium constant for the following formation? Mn<sup>2+</sup> + 2C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66e5_1d9a_93a6_fd88b5379204_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) MnC2O4 + C2O42-
K2 = 1.26 10-2
-What is the equilibrium constant for the following formation? Mn2+ + 2C2O42-
MnC2O4 + C2O42-
K2 = 1.26 10-2
-What is the equilibrium constant for the following formation? Mn2+ + 2C2O42-
![The following questions refer to the following system: 500.0 mL of 0.020 M Mn(NO<sub>3</sub>)<sub>2</sub> are mixed with 1.0 L of 1.0 M Na<sub>2</sub>C<sub>2</sub>O<sub>4</sub>.The oxalate ion,C<sub>2</sub>O<sub>4</sub>,acts as a ligand to form a complex ion with the Mn<sup>2+</sup> ion with a coordination number of two. Mn<sup>2+</sup> + C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4</sub> K<sub>1</sub> = 7.9 \times 10<sup>3</sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup> <sup> </sup> MnC<sub>2</sub>O<sub>4 </sub>+ C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> K<sub>2</sub> = 1.26 \times 10<sup>-</sup><sup>2</sup> -What is the equilibrium constant for the following formation? Mn<sup>2+</sup> + 2C<sub>2</sub>O<sub>4</sub><sup>2</sup><sup>-</sup> <sup> </sup> [Mn(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>2</sup><sup>-</sup>](https://storage.examlex.com/TB6423/11eaa8f0_66e5_44ab_93a6_c10453b99b7d_TB6423_11.jpg) [Mn(C2O4)2]2-
[Mn(C2O4)2]2-
(Multiple Choice)
4.7/5  (36)
(36)
Determine the equilibrium concentration of the chloride ion.
(Multiple Choice)
4.9/5  (38)
(38)
The solubility of La(IO3)3 in a 0.42 M KIO3 solution is 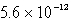 mol/L.Calculate the Ksp for La(IO3)3.
mol/L.Calculate the Ksp for La(IO3)3.
(Multiple Choice)
4.9/5  (32)
(32)
The solubility of silver phosphate,Ag3PO4, at 25°C is 1.60 10-5 mol/L.What is the Ksp for the silver phosphate at 25°C?
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the solubility of Ca3(PO4)2 (Ksp = 1.3 10-32)in a 0.048 M Ca(NO3)2 solution.
(Multiple Choice)
4.8/5  (31)
(31)
An unknown salt,M2Z,has a Ksp of 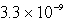 .Calculate the solubility in mol/L of M2Z.
.Calculate the solubility in mol/L of M2Z.
(Multiple Choice)
4.8/5  (34)
(34)
Consider a solution containing the following cations: Na+,Hg2+,Mn2+,Al3+ and Ag+.Treatment of the solution with dilute HCl followed by saturation with H2S results in formation of precipitate(s).Which ions still remain in solution (i.e. ,did not precipitate)?
(Multiple Choice)
4.7/5  (31)
(31)
Calculate the solubility of Ag2CrO4 (Ksp = 9.0 10-12)in a 0.049 M AgNO3 solution.
(Multiple Choice)
4.9/5  (38)
(38)
An industrial plant processes its waste water through a sedimentation tank that removes hazardous metals by precipitating them as insoluble carbonate salts.If sodium carbonate is gradually added to the tank,what would be the order of precipitation of the metals,Pb2+,Cu2+,Hg22+,and Zn2+ if each is 1.0 10-4 M? ( Ksp PbCO3 = 7.4 10-14,Ksp CuCO3 = 1.4 10-10,Ksp Hg2CO3 = 8.9 10-17,and Ksp ZnCO3 = 1.4 10-11)
(Multiple Choice)
4.9/5  (30)
(30)
The solubility of CaSO4 in pure water at 0oC is 1.14 gram(s)per liter.The value of the solubility product is
(Multiple Choice)
4.8/5  (39)
(39)
Given the following values of equilibrium constants: Cu(OH)2(s)  Cu2+(aq)+ 2OH-(aq)
Ksp = 1.60 10-19
Cu(NH3)42+(aq)
Cu2+(aq)+ 2OH-(aq)
Ksp = 1.60 10-19
Cu(NH3)42+(aq)  Cu2+(aq)+ 4NH3(aq)
K = 1.0 10-13
What is the value of the equilibrium constant for the following reaction?
Cu(OH)2(s)+ 4NH3(aq)
Cu2+(aq)+ 4NH3(aq)
K = 1.0 10-13
What is the value of the equilibrium constant for the following reaction?
Cu(OH)2(s)+ 4NH3(aq)  Cu(NH3)42+(aq)+ 2OH-(aq)
Cu(NH3)42+(aq)+ 2OH-(aq)
(Multiple Choice)
4.8/5  (38)
(38)
A solution contains 0.018 moles each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s)precipitates out?
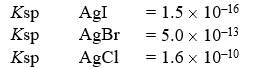
(Multiple Choice)
4.9/5  (40)
(40)
What is the maximum concentration of iodide ions that will precipitate AgI but not PbI2 from a solution that is 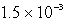 M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
(Multiple Choice)
4.7/5  (33)
(33)
The overall Kf for the complex ion Ag(NH3)2+ is 1.7 107.The Ksp for AgI is 1.5 10-16.What is the molar solubility of AgI in a solution that is 2.0 M in NH3?
(Multiple Choice)
4.8/5  (41)
(41)
In the qualitative analysis scheme for metal ions,how are the Analytical Group II cations separated from the cations of Analytical Groups III-V?
(Multiple Choice)
4.7/5  (29)
(29)
Showing 1 - 20 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)