Deck 16: Manipulation of the Immune Response
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/34
Play
Full screen (f)
Deck 16: Manipulation of the Immune Response
1
In the first few days following organ transplantation, patients are often treated with several doses of an antibody mixture known as anti-thymocyte globulin. The anti-thymocyte globulin is generated by immunizing rabbits or horses with human T cells, to generate antibodies against the human T cell surface molecules. Following the anti-thymocyte globulin treatment, patients are generally put on a sustained regimen of cyclosporin A or other small-molecule immunosuppressive drugs. The immediate post-operative treatment with anti-thymocyte globulin is likely used to:
A) Activate the patient's T cells in vivo to prevent infections
B) Enhance the binding of the T-cell receptor to peptide:MHC on the graft
C) Temporarily deplete the patient's T cells
D) Allow the clinicians to monitor the patient's T cell numbers by flow cytometry
E) Stimulate the development of FoxP3+ Treg cells
A) Activate the patient's T cells in vivo to prevent infections
B) Enhance the binding of the T-cell receptor to peptide:MHC on the graft
C) Temporarily deplete the patient's T cells
D) Allow the clinicians to monitor the patient's T cell numbers by flow cytometry
E) Stimulate the development of FoxP3+ Treg cells
Activate the patient's T cells in vivo to prevent infections
2
Cyclosporin A and rapamycin are each used as T cell immunosuppressants. They share the property of binding to immunophilin molecules in T cells as the initial step in their mechanisms of action. However, in the case of cyclosporin A, the drug:immunophilin complex binds to and inhibits the protein phosphatase calcineurin, whereas the rapamycin:immunophilin complex binds to and inhibitors mTOR. As a consequence,
A) Cyclosporin A, but not rapamycin, blocks cytokine production by T cells.
B) Both cyclosporin A and rapamycin block cytokine production by T cells.
C) Rapamycin, but not cyclosporin A, blocks T cell proliferation.
D) Neither rapamycin nor cyclosporin A block T cell proliferation.
E) Both cyclosporin A and rapamycin inhibit co-stimulatory signaling through CD28 on T cells.
A) Cyclosporin A, but not rapamycin, blocks cytokine production by T cells.
B) Both cyclosporin A and rapamycin block cytokine production by T cells.
C) Rapamycin, but not cyclosporin A, blocks T cell proliferation.
D) Neither rapamycin nor cyclosporin A block T cell proliferation.
E) Both cyclosporin A and rapamycin inhibit co-stimulatory signaling through CD28 on T cells.
Cyclosporin A, but not rapamycin, blocks cytokine production by T cells.
3
Immune responses to tumors have been studied extensively in mice, using transplantable tumors injected into syngeneic mice. The basis for many of these studies is the assumption that the process of tumorigenesis generates mutations in genes encoding self-antigens that would allow the immune system to see these mutant proteins as 'foreign'. In this scenario, the dominant immune response targeting the tumor cells would be mediated by:
A) Antibodies
B) CD4 TH1 cells
C) CD8 T cells
D) NK cells
E) FcR+ phagocytic cells
A) Antibodies
B) CD4 TH1 cells
C) CD8 T cells
D) NK cells
E) FcR+ phagocytic cells
CD8 T cells
4
Mycophenolate mofetil is a cytotoxic drug that is commonly used in patients receiving kidney transplants, as part of a combination therapy with other immunosuppressive drugs. Studies have been performed to assess whether reductions in mycophenolate mofetil dose given to transplant patients starting around 4 months post-transplant have deleterious effects on the transplanted kidney function or on episodes of graft rejection. The motivation for this type of study is:
A) The high expense of combination therapies that require multiple different drugs
B) The side effects of the cytotoxic drug on healthy dividing cells in the body
C) The convenience to the transplant patient of fewer medications to take on a daily basis
D) The off-target effects of mycophenolate mofetil on non-dividing cells
E) The possibility that transplant patients develop an allergic reaction to the mycophenolate mofetil
A) The high expense of combination therapies that require multiple different drugs
B) The side effects of the cytotoxic drug on healthy dividing cells in the body
C) The convenience to the transplant patient of fewer medications to take on a daily basis
D) The off-target effects of mycophenolate mofetil on non-dividing cells
E) The possibility that transplant patients develop an allergic reaction to the mycophenolate mofetil

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
5
The vitamin D3 metabolite 1,25(OH)2D3 binds to the vitamin D receptor (VDR). This complex then functions as a transcriptional regulator. The activation of VDR following treatment of cells with 1,25(OH)2D3 has been found to modulate the activity of antigen-presenting dendritic cells. In one study, dendritic cells were isolated from wild-type mice and activated in vitro by stimulation with LPS plus IFN- in the presence or absence of 1,25(OH)2D3 for 24 hours. These activated dendritic cells were then pulsed with a peptide from the chicken ovalbumin protein (OVA) that binds to MHC class II and is recognized by OT-II CD4 T cells. The peptide-pulsed dendritic cells and T cells were incubated together for 3 days, and then IL-2 levels in the supernatants were measured, as shown in Figure . 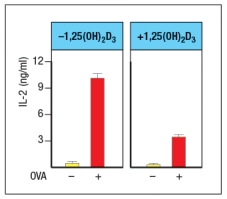
Based on these data, dendritic cells activated in the presence of 1,25(OH)2D3 are likely to show:
A) Reduced MHC class II and reduced B7 expression compared to controls
B) Enhanced expression of IL-12 compared to controls
C) Reduced expression of IL-6 and TNF- compared to controls
D) Increased expression of MHC class I and class II expression compared to controls
E) Reduced expression of proteins involved in antigen presentation compared to controls

Based on these data, dendritic cells activated in the presence of 1,25(OH)2D3 are likely to show:
A) Reduced MHC class II and reduced B7 expression compared to controls
B) Enhanced expression of IL-12 compared to controls
C) Reduced expression of IL-6 and TNF- compared to controls
D) Increased expression of MHC class I and class II expression compared to controls
E) Reduced expression of proteins involved in antigen presentation compared to controls

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
6
A recent strategy showing some promise for the treatment of IgE-mediated allergies is a form of allergen immunotherapy, in which allergic individuals are given 3-4 intradermal injections of soluble peptides. For example, in one study, allergic individuals were injected with a set of 13-17 amino acid long peptides derived from the house dust mite protein antigen; these peptides were selected based on predictions for sequences likely to be MHC class II binding epitopes. Following the treatment, individuals were found to have increased numbers of antigen-specific IL-10-producing CD4 T cells, a finding that correlated with reduced allergic responses to allergen challenge. One of the goals of this therapy might also be to:
A) Reduce the levels of IgE antibodies specific for the allergen
B) Induce type 1 or type 3 immune responses that might inhibit the type 2 allergic responses
C) Induce CD8 T cell responses that would kill allergen specific TH2 cells
D) Induce IgG and IgA antibodies to the allergen
E) Induce apoptosis of allergen-specific effector CD4 T cells
A) Reduce the levels of IgE antibodies specific for the allergen
B) Induce type 1 or type 3 immune responses that might inhibit the type 2 allergic responses
C) Induce CD8 T cell responses that would kill allergen specific TH2 cells
D) Induce IgG and IgA antibodies to the allergen
E) Induce apoptosis of allergen-specific effector CD4 T cells

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
7
Immunotoxin therapy as an anticancer treatment is a focus of current efforts to develop new anticancer drugs. An alternative strategy, known as radioimmunotherapy, involves the conjugation of a tumor-antigen specific antibody to a radioisotope, rather than to a bacterial toxin. One advantage of radioimmunotherapy over that of immunotoxin therapy is that the radioisotope:
A) Will damage neighboring tumor cells in addition to the cell binding the drug
B) Has a finite half-life and will spontaneously lose activity in the patient
C) Is less likely to cause collateral damage to healthy tissues than the toxin
D) Will not cause inflammation due to recognition by PRRs in innate immune cells, but the toxin will
E) Is unable to bind to Fc receptors on phagocytic cells, so will have increased longevity in patients
A) Will damage neighboring tumor cells in addition to the cell binding the drug
B) Has a finite half-life and will spontaneously lose activity in the patient
C) Is less likely to cause collateral damage to healthy tissues than the toxin
D) Will not cause inflammation due to recognition by PRRs in innate immune cells, but the toxin will
E) Is unable to bind to Fc receptors on phagocytic cells, so will have increased longevity in patients

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
8
Corticosteroids are anti-inflammatory drugs that are used to treat individuals with allergies, asthma, autoimmune diseases, or organ transplants. These compounds have a wide range of effects on leukocytes and on inflammatory cytokine production. One common use for corticosteroids is as an inhaled treatment for individuals with asthma. Interestingly, inhaled corticosteroids provide significant benefit to asthma patients with high numbers of eosinophils in their airways, but not to those patients with high numbers of neutrophils, but normal numbers of eosinophils. One reason for this finding may be that:
A) Corticosteroids don't inhibit IL-13 production in the airways
B) Corticosteroids don't inhibit release of IL-33 by airway epithelial cells
C) Corticosteroids induce apoptosis of Treg cells
D) Corticosteroids induce apoptosis of eosinophils
E) Corticosteroids don't work well as combination therapy with other immunosuppressants
A) Corticosteroids don't inhibit IL-13 production in the airways
B) Corticosteroids don't inhibit release of IL-33 by airway epithelial cells
C) Corticosteroids induce apoptosis of Treg cells
D) Corticosteroids induce apoptosis of eosinophils
E) Corticosteroids don't work well as combination therapy with other immunosuppressants

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
9
Immunotherapies aimed at promoting anti-tumor immune responses are being developed for tumors of many different tissue or cell-type origins. Interestingly, some of these approaches, when tested in clinical trials, were found to also cause patients to develop autoimmune symptoms related to their tumor type. For instance, in patients with malignant skin cancer (melanoma), immunotherapy treatment can develop an autoimmune disorder known as vitiligo, in which T cells attack and destroy melanocytes in the skin, causing depigmentation. These findings indicate that, in some individuals the melanoma-specific anti-tumor T cell responses are directed at:
A) Tumor-specific mutated oncogenes
B) Transcription factors not normally expressed in melanocytes
C) Oncogenic proteins encoded by viruses
D) Proteins normally expressed in melanocytes
E) Neoantigens created from expression of abnormally spliced mRNAs
A) Tumor-specific mutated oncogenes
B) Transcription factors not normally expressed in melanocytes
C) Oncogenic proteins encoded by viruses
D) Proteins normally expressed in melanocytes
E) Neoantigens created from expression of abnormally spliced mRNAs

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
10
Abatacept is a biologic drug that is a fusion protein formed from the B7-binding domain of CTLA-4 fused to the human IgG1 constant region. In patients suffering from rheumatoid arthritis (RA), high levels of granzyme B in the synovial fluid of inflamed joints is thought to contribute to joint erosion. When treated with abatacept, a subset of patients shows significant improvement in their RA symptoms together with decreased levels of granzyme B in their affected joints. These data indicate that the granzyme B found in RA patients' inflamed joints is predominantly made by:
A) Antigen-presenting cells such as dendritic cells
B) Activated macrophages
C) Effector T cells
D) FoxP3+ Treg cells
E) Neutrophils
A) Antigen-presenting cells such as dendritic cells
B) Activated macrophages
C) Effector T cells
D) FoxP3+ Treg cells
E) Neutrophils

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
11
Natalizumab is a humanized monoclonal antibody directed at the 4-integrin protein, a subunit of VLA-4, the binding partner of VCAM-1 expressed on endothelium in the central nervous system, and of 4 -integrin, the binding partner of MadCAM-1 expressed on endothelium in the gut. While natalizumab has had great success in alleviating the symptoms of autoimmune diseases such as multiple sclerosis and Crohn's disease, a small number of patients have acquired life threatening infections of the neurotrophic virus, JC. Why are patients on natalizumab particularly susceptible to a virus infection of the central nervous system?

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
12
One of the first biologics developed was a drug known as etanercept (common name, Enbrel) that is a fusion protein formed from the cytokine binding domain of the TNF-receptor fused to the human IgG1 constant region, as shown in Figure Q6). This biologic is used to treat patients with inflammatory diseases such as rheumatoid arthritis, psoriasis, and others. Etanercept was developed to avoid injecting patients with the mouse monoclonal antibody to TNF- , which was available at the time. 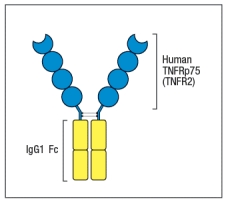
Figure Q6) Etanercept was preferred because:
A) It had a higher affinity for TNF- than the antibody
B) It had a higher valency of binding to TNF- than the antibody
C) It had a longer in vivo half-life than the anti-TNF- antibody
D) It was easier and cheaper to produce than the antibody
E) It would not elicit an anti-drug antibody response

Figure Q6) Etanercept was preferred because:
A) It had a higher affinity for TNF- than the antibody
B) It had a higher valency of binding to TNF- than the antibody
C) It had a longer in vivo half-life than the anti-TNF- antibody
D) It was easier and cheaper to produce than the antibody
E) It would not elicit an anti-drug antibody response

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
13
Alefacept is a fusion protein that contains the CD2-binding domain of LFA3 fused to human IgG1, and it is used to block CD2 function on human T cells. In addition, following in vivo administration, alefacept was also shown to deplete T cells of the effector or memory subsets that express high levels of CD2. Surprisingly, clinical trials data indicated that patients on alefacept still retained responses to vaccination. For example, one set of data showed that patients on alefacept made similar responses as the control group to a vaccine designed to protect against pneumococcal disease, which is composed entirely of bacterial polysaccharide antigens (pneumococcal polysaccharide vaccine; PPV). This normal response to PPV by patients on alefacept is not surprising because:
A) Alefacept is not very effective at blocking T cell responses.
B) CD2 is not required for all T cell responses to vaccination.
C) Alefacept does not deplete all effector or memory T cells, just a subset.
D) T cells are involved in responses to polysaccharide antigens.
E) The PPV was eliciting a recall response in previously exposed individuals.
A) Alefacept is not very effective at blocking T cell responses.
B) CD2 is not required for all T cell responses to vaccination.
C) Alefacept does not deplete all effector or memory T cells, just a subset.
D) T cells are involved in responses to polysaccharide antigens.
E) The PPV was eliciting a recall response in previously exposed individuals.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
14
To study the immune responses that provide protection against tumor growth in mice, some investigations have used the EL4 mouse thymoma line. This tumor cell line was derived from a C57BL/6 mouse, and represents a type of T cell lymphoma. When transplanted into Rag-deficient mice, the mice rapidly succumb to the tumor. This is also the case when the mice receive wild-type C57BL/6 T cells the day before tumor cell injection. However, Rag-deficient mice were protected when the transferred T cells came from mice that lacked expression of one cytokine receptor, as shown in Figure . Name one likely candidate for the cytokine receptor that is knocked out in the T cells that are able to protect the mice. 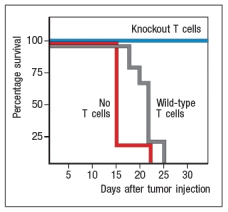


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
15
One successful cancer vaccine targets human papilloma virus serotypes (HPV-16, -18) associated with the majority of cases of cervical cancer. Follow-up studies performed on large cohorts of women who received this vaccine showed long-lasting (~10 years) serum antibody responses to the vaccine. How does this vaccine prevent cancer?

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
16
An area of cancer immunotherapy that is undergoing rapid development is the use of agents that function as checkpoint blockaders. These agents work by interfering with receptors on T cells that normally induce inhibitory signals that suppress T cell responses. For example, one clinical trial tested the effects of a drug known as ipilimumab, which is a human monoclonal antibody that binds to and inhibits CTLA-4, on patients with metastatic melanoma, a deadly form of skin cancer. A simplified version of these data are shown in Figure Q20). In this case, one group of patients received a peptide vaccine, comprised of a mixture of HLA class I-binding peptides derived from the melanoma antigen, gp100. A second group of patients received ipilimumab, and a third group received the gp100 peptide vaccine + ipilimumab. 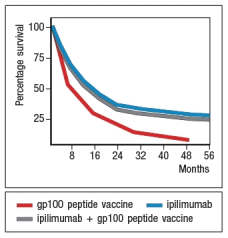 survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:
survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:
A) That it promotes the development of effector T cells from tumor-specific naive T cells
B) That it allows T cells already present in the patient to kill tumor cells more effectively
C) That it promotes the activation of CD4 instead of CD8 T cells specific for the tumor
D) To prevent the immunosuppressive environment of the tumor from killing T cells
E) To act directly on the tumor cells, similar to a cytotoxic chemotherapeutic drug
 survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:
survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:A) That it promotes the development of effector T cells from tumor-specific naive T cells
B) That it allows T cells already present in the patient to kill tumor cells more effectively
C) That it promotes the activation of CD4 instead of CD8 T cells specific for the tumor
D) To prevent the immunosuppressive environment of the tumor from killing T cells
E) To act directly on the tumor cells, similar to a cytotoxic chemotherapeutic drug

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
17
Type 1 diabetes has traditionally been considered a T-cell-mediated autoimmune disease. However, recent studies have shown that treatment of newly diagnosed type 1 diabetes patients with rituximab (ant-CD20 antibody) had a significant effect in slowing down the progression of their disease. These results indicate that autoantibodies likely contribute to the process of pancreatic -islet cell destruction in these patients.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
18
In 2015, the US FDA approved the use of secukinumab for the treatment of psoriasis, a disease caused by chronic inflammation in the skin. Secukinumab is fully human monoclonal antibody against IL-17A. Clinical trials of this treatment indicated an increased rate of infections in patients on secukinumab, the majority of which were upper respiratory tract infections caused by viral or bacterial pathogens. For many of these infections, this side effect of secukinumab can be explained by:
A) Poor recruitment of neutrophils to infected airways
B) Lack of mucus production by airway epithelial cells
C) Poor recruitment of eosinophils to infected airways
D) Enhanced production of antimicrobial peptides by airway epithelial cells
E) Loss of tight junctions between airway epithelial cells
A) Poor recruitment of neutrophils to infected airways
B) Lack of mucus production by airway epithelial cells
C) Poor recruitment of eosinophils to infected airways
D) Enhanced production of antimicrobial peptides by airway epithelial cells
E) Loss of tight junctions between airway epithelial cells

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
19
CAR T cells have shown remarkable efficacy in treating hematological malignancies . 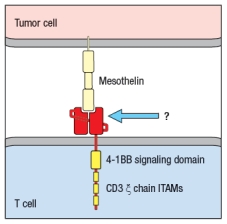 Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:
Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:
A) A T-cell receptor specific for mesothelin
B) The ligand that normally binds to mesothelin
C) The Fab fragment of an anti-mesothelin antibody
D) A co-stimulatory receptor signaling domain
E) The CD3 signaling components of the T-cell receptor
 Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:
Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:A) A T-cell receptor specific for mesothelin
B) The ligand that normally binds to mesothelin
C) The Fab fragment of an anti-mesothelin antibody
D) A co-stimulatory receptor signaling domain
E) The CD3 signaling components of the T-cell receptor

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
20
The first JAK kinase inhibitor to be developed, tofacitinib, inhibits JAK1 and JAK3. These two JAK kinases are required for the signaling pathways induced by multiple cytokines, including all of the receptors that share the cytokine receptor common gamma chain ( c; includes the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), all of the receptors that share gp130 (includes the receptor for IL-6), the receptors for GM-CSF, IL-12, and IL-23, and both type I and type II (i.e., IFN- ) interferon receptors. Tofacitinib has recently been shown to be effective in the treatment of severe rheumatoid arthritis, an autoimmune disease characterized by inflammation of the joints with prominent inflammatory cell infiltrates, autoantibodies, and eventual cartilage and bone destruction. A likely explanation for the therapeutic benefits of tofacitinib in this disease is:
A) Its ability to inhibit the T cell functions that eventually lead to other disease symptoms
B) Its ability to prevent T cell help for B cells producing autoantibodies
C) Its ability to block migration of inflammatory cells into joints
D) Its ability to block innate as well as adaptive immune responses
E) Its ability to prevent T cell proliferation by blocking IL-2 receptor signaling
A) Its ability to inhibit the T cell functions that eventually lead to other disease symptoms
B) Its ability to prevent T cell help for B cells producing autoantibodies
C) Its ability to block migration of inflammatory cells into joints
D) Its ability to block innate as well as adaptive immune responses
E) Its ability to prevent T cell proliferation by blocking IL-2 receptor signaling

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
21
As of 2014, a total of 452 clinical studies of peptide-based vaccines were registered with the US federal government clinical trials database. Of these, none have progressed to market, and only a small number (12) made it to phase III clinical trials; furthermore, of these phase III trials, none were for vaccines directed against infectious diseases. The lack of success of peptide vaccines against pathogenic infections is due to the fact that antibodies against a single epitope on a pathogen are rarely protective.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
22
Most vaccines currently administered are delivered by intramuscular injection. Yet the pathogenic organisms these vaccines aim to protect against usually enter the body by a different route. For instance, many viruses infect us via the respiratory tract, and many bacterial pathogens infect via the gastrointestinal tract. One major advantage of delivering vaccines against these organisms via their normal route of infection would be:
A) That the vaccines would no longer require the incorporation of an adjuvant in their composition
B) That the vaccines would elicit IgA antibodies in addition to IgG antibodies
C) That the vaccines would no longer require several boosters to elicit protective responses
D) That the vaccines would be more stable and not require refrigeration
E) That the vaccines would be safer and have fewer side effects than injected vaccines
A) That the vaccines would no longer require the incorporation of an adjuvant in their composition
B) That the vaccines would elicit IgA antibodies in addition to IgG antibodies
C) That the vaccines would no longer require several boosters to elicit protective responses
D) That the vaccines would be more stable and not require refrigeration
E) That the vaccines would be safer and have fewer side effects than injected vaccines

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
23
Effective vaccines have been developed against a number of deadly diseases, including viral diseases such as measles, mumps, polio, and smallpox, as well as several bacterial infections. However, there remain several important infectious diseases for which vaccines are currently lacking. One important aspect of developing effective vaccines against these pathogens is:
A) The discovery of an attenuated form of the pathogenic organism
B) The purification of surface components of the pathogen
C) A clear understanding of the immune mechanisms required to eliminate the infection
D) The purification of toxins made by the pathogen
E) The development of more effective adjuvants to include in the vaccine
A) The discovery of an attenuated form of the pathogenic organism
B) The purification of surface components of the pathogen
C) A clear understanding of the immune mechanisms required to eliminate the infection
D) The purification of toxins made by the pathogen
E) The development of more effective adjuvants to include in the vaccine

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
24
One clinical trial performed in the early 2000s tested whether addition of unmethylated CpG oligonucleotides (CpG ODN) to the hepatitis B vaccine improved immune responses in volunteer vaccinees. The normal vaccine consists of purified recombinant hepatitis B surface antigen protein mixed with alum. Half of the subjects received this original vaccine, and half received the original vaccine with added CpG ODN. The study found that individuals receiving the vaccine plus CpG ODN showed more rapid and robust antibody responses, and a trend toward enhanced CD8 cytotoxic T cell responses compared to the vaccine alone group. As expected, the group receiving the vaccine plus CpG ODN also:
A) Reported more frequent side effects of flu-like symptoms, such as mild fever
B) Showed an increased TH17 response to the Hep B surface antigen
C) Exhibited lower levels of type I interferon in their sera than the control group
D) Had reduced numbers of activated dendritic cells in their blood
E) Had similar proportions of the different antibody isotypes directed against Hep B surface antigen
A) Reported more frequent side effects of flu-like symptoms, such as mild fever
B) Showed an increased TH17 response to the Hep B surface antigen
C) Exhibited lower levels of type I interferon in their sera than the control group
D) Had reduced numbers of activated dendritic cells in their blood
E) Had similar proportions of the different antibody isotypes directed against Hep B surface antigen

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
25
Autoimmune diseases are characterized by an adaptive immune response directed at self-tissue or self-antigens. One hallmark of these diseases is their responsiveness to treatments that inhibit B or T lymphocytes. A set of diseases known as autoinflammatory diseases are, instead, caused by aberrant activation of innate immune responses. Some of these are monogenic inherited disorders, for example, a spectrum of diseases resulting from mutations in the innate sensor protein, NLRP3. These diseases are inherited as autosomal dominant diseases, and are due to gain-of-function mutations in NLRP3, a key component of the inflammasome. Altogether, these disorders are known as CAPS, for cryopyrin-associated periodic syndrome, as they generally cause periodic episodes of fever and inflammation. Common symptoms of these disorders include severe skin rashes, fevers, joint disease, and central nervous system disease. A biologic has been developed to treat patients with CAPS, with remarkable success. Initial studies first examined the responses of peripheral blood cells from CAPS patients to this biologic, compared to controls. For these experiments, peripheral blood mononuclear cells (all white blood cells) were cultured for 48 hours, without stimulation, and the cytokine levels in the supernatants were then measured by ELISA. These data are shown in Figure. 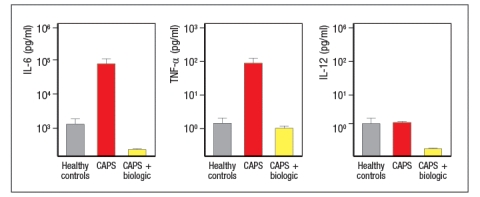
a) Based on these data, which cells in the peripheral blood of CAPS patients are spontaneously producing cytokines?
b) Based on the fact that CAPS patients have a mutation in a single gene, NLRP3, propose an explanation for the cytokine data shown above
Next, peripheral blood mononuclear cells from controls or CAPS patients were stimulated in vitro with LPS alone, and protein was isolated from cell lysates and cell supernatants, and immunoblotted as shown in Figure
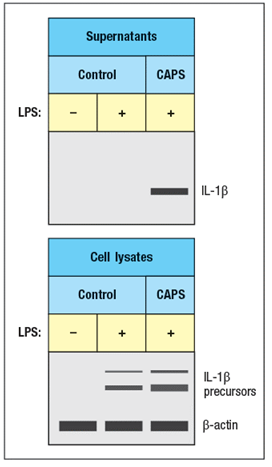
c) What do these results indicate about the consequences of the gain-of-function NLRP3 mutations in patients with CAPS?on normal healthy peripheral blood mononuclear cells, cells from healthy controls were cultured in vitro for 48 hours in the presence or absence of IL-1 . Cytokines in the supernatants were then analyzed, in comparison to supernatants from cultured CAPS patients' cells. For these studies, the CAPS patients' cells were cultured without stimulation and in the absence of any biologic treatment. These data are shown in Figure.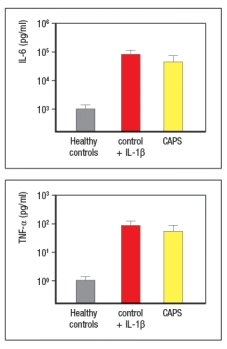
In addition, serum cytokine levels from CAPS patients were measured before and after treatment with the biologic, and the results are shown in Table .
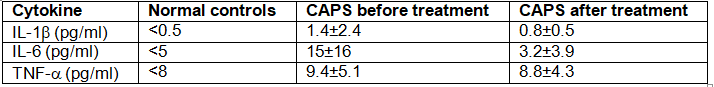
d) What do these data suggest a possible explanation for the inflammatory syndrome seen in CAPS patients?
e) Based on all of these data, what is the likely target of the biologic used to treat the CAPS patients?

a) Based on these data, which cells in the peripheral blood of CAPS patients are spontaneously producing cytokines?
b) Based on the fact that CAPS patients have a mutation in a single gene, NLRP3, propose an explanation for the cytokine data shown above
Next, peripheral blood mononuclear cells from controls or CAPS patients were stimulated in vitro with LPS alone, and protein was isolated from cell lysates and cell supernatants, and immunoblotted as shown in Figure

c) What do these results indicate about the consequences of the gain-of-function NLRP3 mutations in patients with CAPS?on normal healthy peripheral blood mononuclear cells, cells from healthy controls were cultured in vitro for 48 hours in the presence or absence of IL-1 . Cytokines in the supernatants were then analyzed, in comparison to supernatants from cultured CAPS patients' cells. For these studies, the CAPS patients' cells were cultured without stimulation and in the absence of any biologic treatment. These data are shown in Figure.

In addition, serum cytokine levels from CAPS patients were measured before and after treatment with the biologic, and the results are shown in Table .

d) What do these data suggest a possible explanation for the inflammatory syndrome seen in CAPS patients?
e) Based on all of these data, what is the likely target of the biologic used to treat the CAPS patients?

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
26
Studies in mice have revealed important features about some chronic infections. In the case of mice infected with a persistent strain of lymphocytic choriomeningitis virus (LCMV), these studies showed that a majority of the virus-specific T cells were eliminated from the mice over time; however, some virus-specific CD8 T cells persisted but were unable to eradicate the viral infection. These remaining cells expressed high levels of several inhibitory receptors, including PD-1. One possible treatment for these mice to promote their ability to clear this chronic infection would be:
A) Immunization with an inactivated preparation of LCMV
B) Administration of anti-PD-1 antibody
C) Administration of an antiviral drug that would block LCMV replication
D) Immunization with a purified surface antigen from LCMV
E) Stimulation with a TLR7 or TLR9 ligand to induce an interferon response
A) Immunization with an inactivated preparation of LCMV
B) Administration of anti-PD-1 antibody
C) Administration of an antiviral drug that would block LCMV replication
D) Immunization with a purified surface antigen from LCMV
E) Stimulation with a TLR7 or TLR9 ligand to induce an interferon response

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
27
Respiratory syncytial virus (RSV) is a common pathogen that nearly all children are infected with by age 2. It is currently the leading cause of hospitalization of infants. In 1966 a vaccine against RSV was developed and tested in children. The vaccine was made by inactivating RSV virions with formalin, and then injecting the inactivated virus into recipients; the vaccine was known as FIRSV . The top graph shows the results of an assay used to assess the relative affinities of antibodies in sera from immunized mice. Sera were isolated from mice immunized with live RSV versus FIRSV, and the isolated sera were tested for binding to immobilized RSV particles. The graph depicts the percentage of IgG antibody remaining bound to virus after washing in increasing concentrations of urea. The higher the affinity, the greater the amount of antibody that remains bound to antigen as the urea concentration increases. The bottom graph shows the titers of neutralizing antibody in the serum of mice immunized with FIRSV or with live RSV, compared to an unimmunized control. In this case, sera at different dilutions are mixed with live RSV, and then the mixture is tested for the amount of infectious virus remaining in solution. The log2 PRNT (50% plaque reduction) indicates the amount the serum can be diluted and still retain the ability to neutralize 50% of the virus particles. Based on these data, what is the likely explanation for the failure of the FIRSV vaccine to protect recipients? 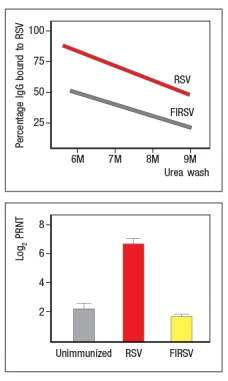


Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
28
Conjugate vaccines, such as those developed against H. influenzae type B and N. meningitidis type C, provide protection in small children and infants that are unable to make antibody responses to isolated polysaccharide antigens. However, one group of individuals that would generate antibody responses to the purified polysaccharide vaccine that are as effective as the antibodies they would make in response to the conjugate vaccine is:
A) Older individuals, such as those over the age of 50
B) Infants with X-linked SCID
C) Individuals with X-linked agammaglobulinemia
D) Individuals with hyper-IgM syndrome due to CD40-ligand deficiency
E) Individuals with chronic granulomatous disease due to deficiencies in integrin expression
A) Older individuals, such as those over the age of 50
B) Infants with X-linked SCID
C) Individuals with X-linked agammaglobulinemia
D) Individuals with hyper-IgM syndrome due to CD40-ligand deficiency
E) Individuals with chronic granulomatous disease due to deficiencies in integrin expression

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
29
The US Department of Health and Human Services has a stated goal for the seasonal influenza vaccine of vaccinating 80% of healthy (i.e., low-risk) individuals. This vaccine is formulated each year from the serotypes of influenza likely to be circulating in the population during the coming flu season. The reason this goal is not 100% of individuals is because:
A) It is not feasible to expect 100% of healthy individuals to get the flu vaccine every year.
B) Individuals who had the flu vaccine the year before will already be protected.
C) Individuals who are healthy need not be too concerned about getting infected with flu.
D) Healthy individuals are unlikely to spread the virus to others.
E) Unvaccinated individuals are protected when 80% of people around them are vaccinated.
A) It is not feasible to expect 100% of healthy individuals to get the flu vaccine every year.
B) Individuals who had the flu vaccine the year before will already be protected.
C) Individuals who are healthy need not be too concerned about getting infected with flu.
D) Healthy individuals are unlikely to spread the virus to others.
E) Unvaccinated individuals are protected when 80% of people around them are vaccinated.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
30
Currently, the only licensed vaccine against Mycobacterium tuberculosis (Mtb) is the attenuated strain, related to Mtb, known as BCG. While BCG protects some populations against some strains of Mtb, an improved vaccine for this deadly disease is needed. One recent effort to generate a better vaccine against Mtb is to engineer BCG to have improved immunogenicity in vaccinated individuals. BCG, like Mtb, infects macrophages, replicating in the macrophage phagosomes. Both BCG and Mtb prevent phagosome acidification and fusion with lysozomes, thereby preventing macrophages from efficiently killing the ingested bacteria. In addition, antigens from the bacteria are not found in the macrophage cytosol. To alter this lifestyle, BCG was engineered to express the listeriolysin protein from Listeria monocytogenes. This enzyme lyses the phagosomal membrane allowing antigens to escape into the cytosol. In addition, a mutation was generated in this same BCG to inactivate the bacterial urease, an enzyme that prevents acidification of phagosomes carrying ingested bacteria. This modified BCG was called rBCG, for recombinant BCG. To test whether rBCG induced more potent protection than the original vaccine strain, mice were immunized with BCG or rBCG, or left unimmunized, and then challenged with virulent Mtb. Titers of Mtb (CFU/lung) were then examined over time after challenge, as shown in Figure Q25). 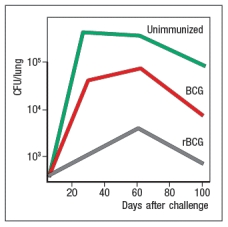 Figure Q25) Based on these data, rBCG likely induced:
Figure Q25) Based on these data, rBCG likely induced:
A) Increased cytolytic CD8 T cells capable of killing Mtb-infected macrophages
B) Increased neutralizing antibody responses to Mtb
C) Increased numbers of CD4 TFH cells to Mtb peptides
D) Increased activation of NK cells capable of killing Mtb-infected macrophages
E) A decrease in the ability of Mtb to infect macrophages in the vaccinated mice
 Figure Q25) Based on these data, rBCG likely induced:
Figure Q25) Based on these data, rBCG likely induced:A) Increased cytolytic CD8 T cells capable of killing Mtb-infected macrophages
B) Increased neutralizing antibody responses to Mtb
C) Increased numbers of CD4 TFH cells to Mtb peptides
D) Increased activation of NK cells capable of killing Mtb-infected macrophages
E) A decrease in the ability of Mtb to infect macrophages in the vaccinated mice

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
31
One early study on DNA vaccines examined the CD8 T cell response to the plasmid-encoded antigenic protein following subcutaneous immunization of mice with the plasmid DNA. This study used bone marrow chimeras to distinguish the MHC class I alleles expressed on bone marrow-derived cells from those expressed on all other mouse cells and tissues. When analyzed, the data showed that antigen-specific CD8 T cell responses were restricted to the MHC class I alleles derived from the donor bone marrow. These findings indicate that a key step in DNA vaccination is:
A) The uptake of the plasmid DNA by antigen-presenting cells in the skin
B) The expression and secretion of the plasmid DNA-encoded antigenic protein by skin keratinocytes
C) The stimulation of keratinocytes to produce inflammatory cytokines in response to the plasmid DNA-encoded antigenic protein
D) The presentation of peptides derived from the antigenic protein on MHC class I molecules of skin-resident dendritic cells
E) The trafficking of the plasmid DNA-encoded antigenic protein to the draining lymph nodes of the skin for uptake by phagocytic cells
A) The uptake of the plasmid DNA by antigen-presenting cells in the skin
B) The expression and secretion of the plasmid DNA-encoded antigenic protein by skin keratinocytes
C) The stimulation of keratinocytes to produce inflammatory cytokines in response to the plasmid DNA-encoded antigenic protein
D) The presentation of peptides derived from the antigenic protein on MHC class I molecules of skin-resident dendritic cells
E) The trafficking of the plasmid DNA-encoded antigenic protein to the draining lymph nodes of the skin for uptake by phagocytic cells

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
32
Large clinical trials have been performed to compare the vaccine efficacy of the live-attenuated influenza virus vaccine with that of the inactivated virus vaccine. In all, data from over 25,000 children aged 6-71 months were analyzed. The conclusion was that the children who received the live-attenuated vaccine reported 50% fewer cases of influenza than those who received the inactivated vaccine. One difference in the immune response elicited by the live-attenuated versus the inactivated vaccine is:
A) The live-attenuated vaccine elicits higher antibody titers than the inactivated vaccine.
B) The live-attenuated vaccine elicits antibodies with higher affinities than the inactivated vaccine.
C) The live-attenuated vaccine elicits increased numbers of CD4 TFH cells than the inactivated vaccine.
D) The live-attenuated vaccine elicits antiviral CD8 effector responses but the inactivated vaccine does not.
E) The live-attenuated vaccine induces antibodies of the IgA isotype, but the inactivated vaccine does not.
A) The live-attenuated vaccine elicits higher antibody titers than the inactivated vaccine.
B) The live-attenuated vaccine elicits antibodies with higher affinities than the inactivated vaccine.
C) The live-attenuated vaccine elicits increased numbers of CD4 TFH cells than the inactivated vaccine.
D) The live-attenuated vaccine elicits antiviral CD8 effector responses but the inactivated vaccine does not.
E) The live-attenuated vaccine induces antibodies of the IgA isotype, but the inactivated vaccine does not.

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
33
Safety concerns in the latter part of the twentieth century prompted the development of an acellular formulation of a vaccine directed against Bordetella pertussis, the causative agent of whooping cough. This acellular vaccine, made from a mixture of four
A) A robust antibody response comprised primarily of IgA
B) A robust systemic antibody response comprised primarily of IgG
C) A strong inflammatory response leading to septic shock
D) A robust cellular response comprised primarily of TH17 cells
E) A robust cellular response comprised primarily of TFH cells
A) A robust antibody response comprised primarily of IgA
B) A robust systemic antibody response comprised primarily of IgG
C) A strong inflammatory response leading to septic shock
D) A robust cellular response comprised primarily of TH17 cells
E) A robust cellular response comprised primarily of TFH cells

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck
34
Early efforts to develop a vaccine against respiratory syncytial virus (RSV) using inactivated virus were unsuccessful. Antibody responses to the vaccine were poor, and were not protective against the natural infection. Studies in mice revealed a likely explanation for the poor performance of the inactivated RSV vaccine. For these studies, inactivated RSV was generated by UV-treatment of the virus, and was referred to as UVRSV. This UVRSV was compared to live RSV in a number of assays. First, mice were immunized by footpad (intradermal, also known as subcutaneous) injection of either UVRSV or live RSV, and dendritic cells from the draining lymph node were isolated 24 hours later. Cell lysates from these dendritic cells were prepared, and were immunoblotted for the levels of I B protein, the inhibitory subunit of the cytosolic complex of NF B that is degraded following stimulation of receptors that activate NF B. A loading control of -actin is shown to indicate comparable amounts of total protein in each sample. 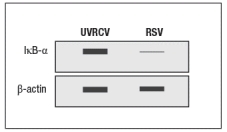
a) Given these data, name two classes of receptors in dendritic cells that might account for the difference seen in the immunoblot.
To examine further the different effects of the UVRSV versus live RSV immunization on dendritic cells, the dendritic cells were isolated from draining lymph nodes 24 hours after immunization and stained for surface expression of CD40 and the co-stimulatory B7 receptors. These data are shown in Figure as fold-increase over the levels of expression of these molecules detected on dendritic cells from unimmunized mice.
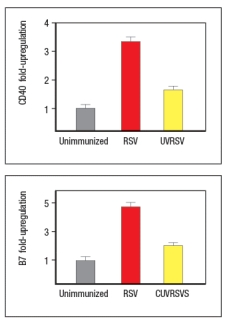
Next, mice were immunized with live RSV or UVRSV, and CD4 T cells were isolated from draining lymph nodes on various days post-immunization. The T cells were examined for IFN- production in response to viral antigens mixed with antigen-presenting-cells by ELISPOT, and the numbers of responding cells at each time point were graphed, as shown in Figure.
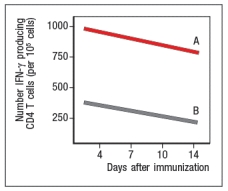
b) Which data (A or B) represents the live RSV versus the UVRSV, and why?
To determine which dendritic cell pathway might be important for the improved responses to live RSV compared to UVRSV, live RSV was used to immunize mice with reduced expression of the adapter protein, MyD88, and the responses of the Myd88+/- mice were compared to wild-type immunized mice. In these studies, anti-RSV antibodies in sera of immunized mice were tested for their ability to neutralize infectious virus in an in vitro assay, as shown in Figure. Log2PRNT indicates the amount the sera can be diluted before it loses the ability to neutralize 50% of the infectious virus in each sample, on a log2 scale (i.e., the higher the value the more potent the sera is for neutralizing virus).
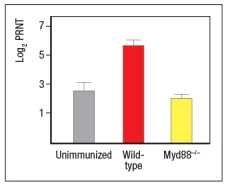
c) Based on these data, what is the likely identity of the receptor family required for live RSV immunization to produce neutralizing antibodies to RSV?
To determine whether the pathway identified above was responsible for the more robust response to live RSV compared to UVRSV, mice were immunized with UVRSV alone, UVRSV in combination with alum, or UVRSV in combination with synthetic double-stranded RNA (poly I:C) plus synthetic single-stranded RNA (poly U). The sera from these mice were then tested for their ability to neutralize infectious RSV, in comparison to sera from unimmunized mice or mice immunized with live RSV, as shown in .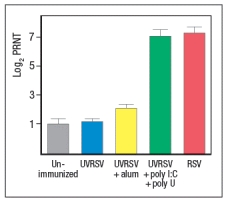
d) Do these data support or refute your answer to question (c) above? Do they implicate any specific receptors in the pathway required to generate a robust neutralizing antibody response to RSV?

a) Given these data, name two classes of receptors in dendritic cells that might account for the difference seen in the immunoblot.
To examine further the different effects of the UVRSV versus live RSV immunization on dendritic cells, the dendritic cells were isolated from draining lymph nodes 24 hours after immunization and stained for surface expression of CD40 and the co-stimulatory B7 receptors. These data are shown in Figure as fold-increase over the levels of expression of these molecules detected on dendritic cells from unimmunized mice.

Next, mice were immunized with live RSV or UVRSV, and CD4 T cells were isolated from draining lymph nodes on various days post-immunization. The T cells were examined for IFN- production in response to viral antigens mixed with antigen-presenting-cells by ELISPOT, and the numbers of responding cells at each time point were graphed, as shown in Figure.

b) Which data (A or B) represents the live RSV versus the UVRSV, and why?
To determine which dendritic cell pathway might be important for the improved responses to live RSV compared to UVRSV, live RSV was used to immunize mice with reduced expression of the adapter protein, MyD88, and the responses of the Myd88+/- mice were compared to wild-type immunized mice. In these studies, anti-RSV antibodies in sera of immunized mice were tested for their ability to neutralize infectious virus in an in vitro assay, as shown in Figure. Log2PRNT indicates the amount the sera can be diluted before it loses the ability to neutralize 50% of the infectious virus in each sample, on a log2 scale (i.e., the higher the value the more potent the sera is for neutralizing virus).

c) Based on these data, what is the likely identity of the receptor family required for live RSV immunization to produce neutralizing antibodies to RSV?
To determine whether the pathway identified above was responsible for the more robust response to live RSV compared to UVRSV, mice were immunized with UVRSV alone, UVRSV in combination with alum, or UVRSV in combination with synthetic double-stranded RNA (poly I:C) plus synthetic single-stranded RNA (poly U). The sera from these mice were then tested for their ability to neutralize infectious RSV, in comparison to sera from unimmunized mice or mice immunized with live RSV, as shown in .

d) Do these data support or refute your answer to question (c) above? Do they implicate any specific receptors in the pathway required to generate a robust neutralizing antibody response to RSV?

Unlock Deck
Unlock for access to all 34 flashcards in this deck.
Unlock Deck
k this deck


