Exam 16: Manipulation of the Immune Response
An area of cancer immunotherapy that is undergoing rapid development is the use of agents that function as checkpoint blockaders. These agents work by interfering with receptors on T cells that normally induce inhibitory signals that suppress T cell responses. For example, one clinical trial tested the effects of a drug known as ipilimumab, which is a human monoclonal antibody that binds to and inhibits CTLA-4, on patients with metastatic melanoma, a deadly form of skin cancer. A simplified version of these data are shown in Figure Q20). In this case, one group of patients received a peptide vaccine, comprised of a mixture of HLA class I-binding peptides derived from the melanoma antigen, gp100. A second group of patients received ipilimumab, and a third group received the gp100 peptide vaccine + ipilimumab. 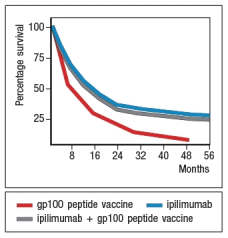 survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:
survival of ~20% of the patients receiving ipilimumab. However, based on the similarity in the data for the group of patients receiving ipilimumab plus the gp100 peptide vaccine versus those receiving ipilimumab alone, the most likely affect of the ipilimumab is:
B
To study the immune responses that provide protection against tumor growth in mice, some investigations have used the EL4 mouse thymoma line. This tumor cell line was derived from a C57BL/6 mouse, and represents a type of T cell lymphoma. When transplanted into Rag-deficient mice, the mice rapidly succumb to the tumor. This is also the case when the mice receive wild-type C57BL/6 T cells the day before tumor cell injection. However, Rag-deficient mice were protected when the transferred T cells came from mice that lacked expression of one cytokine receptor, as shown in Figure . Name one likely candidate for the cytokine receptor that is knocked out in the T cells that are able to protect the mice. 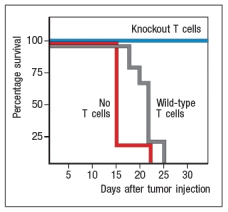
TGF- receptor. This study was actually performed by generating T cells that expressed a dominant-negative form of the TGF- receptor, rather than a TGF- receptor knockout. Nonetheless, the results showed that T cells that are unable to respond to TGF- will eradicate the EL4 tumor cells in Rag-deficient mice, thereby protecting the mice from the lethal growth of this tumor. These results imply that the tumor is producing TGF- , or that it recruits TGF- -producing cells into its environment. TGF- will inhibit the development of effector T cells capable of eradicating the tumor. If the T cells are unable to respond to TGF- , they are able to differentiate into tumor-eradicating effector cells.
Another possible answer is IL-10 receptor; however, data on the role of IL-10 in suppressing anti-tumor T cell responses in this system are not available.
Currently, the only licensed vaccine against Mycobacterium tuberculosis (Mtb) is the attenuated strain, related to Mtb, known as BCG. While BCG protects some populations against some strains of Mtb, an improved vaccine for this deadly disease is needed. One recent effort to generate a better vaccine against Mtb is to engineer BCG to have improved immunogenicity in vaccinated individuals. BCG, like Mtb, infects macrophages, replicating in the macrophage phagosomes. Both BCG and Mtb prevent phagosome acidification and fusion with lysozomes, thereby preventing macrophages from efficiently killing the ingested bacteria. In addition, antigens from the bacteria are not found in the macrophage cytosol. To alter this lifestyle, BCG was engineered to express the listeriolysin protein from Listeria monocytogenes. This enzyme lyses the phagosomal membrane allowing antigens to escape into the cytosol. In addition, a mutation was generated in this same BCG to inactivate the bacterial urease, an enzyme that prevents acidification of phagosomes carrying ingested bacteria. This modified BCG was called rBCG, for recombinant BCG. To test whether rBCG induced more potent protection than the original vaccine strain, mice were immunized with BCG or rBCG, or left unimmunized, and then challenged with virulent Mtb. Titers of Mtb (CFU/lung) were then examined over time after challenge, as shown in Figure Q25). 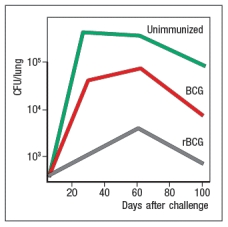 Figure Q25) Based on these data, rBCG likely induced:
Figure Q25) Based on these data, rBCG likely induced:
A
In 2015, the US FDA approved the use of secukinumab for the treatment of psoriasis, a disease caused by chronic inflammation in the skin. Secukinumab is fully human monoclonal antibody against IL-17A. Clinical trials of this treatment indicated an increased rate of infections in patients on secukinumab, the majority of which were upper respiratory tract infections caused by viral or bacterial pathogens. For many of these infections, this side effect of secukinumab can be explained by:
Corticosteroids are anti-inflammatory drugs that are used to treat individuals with allergies, asthma, autoimmune diseases, or organ transplants. These compounds have a wide range of effects on leukocytes and on inflammatory cytokine production. One common use for corticosteroids is as an inhaled treatment for individuals with asthma. Interestingly, inhaled corticosteroids provide significant benefit to asthma patients with high numbers of eosinophils in their airways, but not to those patients with high numbers of neutrophils, but normal numbers of eosinophils. One reason for this finding may be that:
Respiratory syncytial virus (RSV) is a common pathogen that nearly all children are infected with by age 2. It is currently the leading cause of hospitalization of infants. In 1966 a vaccine against RSV was developed and tested in children. The vaccine was made by inactivating RSV virions with formalin, and then injecting the inactivated virus into recipients; the vaccine was known as FIRSV . The top graph shows the results of an assay used to assess the relative affinities of antibodies in sera from immunized mice. Sera were isolated from mice immunized with live RSV versus FIRSV, and the isolated sera were tested for binding to immobilized RSV particles. The graph depicts the percentage of IgG antibody remaining bound to virus after washing in increasing concentrations of urea. The higher the affinity, the greater the amount of antibody that remains bound to antigen as the urea concentration increases. The bottom graph shows the titers of neutralizing antibody in the serum of mice immunized with FIRSV or with live RSV, compared to an unimmunized control. In this case, sera at different dilutions are mixed with live RSV, and then the mixture is tested for the amount of infectious virus remaining in solution. The log2 PRNT (50% plaque reduction) indicates the amount the serum can be diluted and still retain the ability to neutralize 50% of the virus particles. Based on these data, what is the likely explanation for the failure of the FIRSV vaccine to protect recipients? 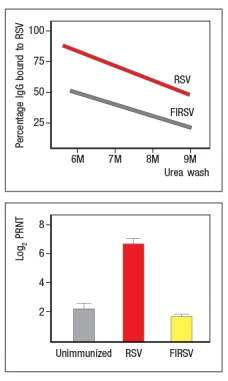
Most vaccines currently administered are delivered by intramuscular injection. Yet the pathogenic organisms these vaccines aim to protect against usually enter the body by a different route. For instance, many viruses infect us via the respiratory tract, and many bacterial pathogens infect via the gastrointestinal tract. One major advantage of delivering vaccines against these organisms via their normal route of infection would be:
Large clinical trials have been performed to compare the vaccine efficacy of the live-attenuated influenza virus vaccine with that of the inactivated virus vaccine. In all, data from over 25,000 children aged 6-71 months were analyzed. The conclusion was that the children who received the live-attenuated vaccine reported 50% fewer cases of influenza than those who received the inactivated vaccine. One difference in the immune response elicited by the live-attenuated versus the inactivated vaccine is:
CAR T cells have shown remarkable efficacy in treating hematological malignancies . 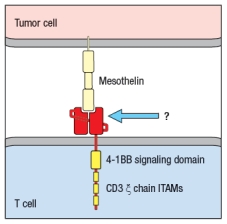 Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:
Figure Q17) In this cartoon, the component of the CAR indicated by the arrow is composed of:
Studies in mice have revealed important features about some chronic infections. In the case of mice infected with a persistent strain of lymphocytic choriomeningitis virus (LCMV), these studies showed that a majority of the virus-specific T cells were eliminated from the mice over time; however, some virus-specific CD8 T cells persisted but were unable to eradicate the viral infection. These remaining cells expressed high levels of several inhibitory receptors, including PD-1. One possible treatment for these mice to promote their ability to clear this chronic infection would be:
Mycophenolate mofetil is a cytotoxic drug that is commonly used in patients receiving kidney transplants, as part of a combination therapy with other immunosuppressive drugs. Studies have been performed to assess whether reductions in mycophenolate mofetil dose given to transplant patients starting around 4 months post-transplant have deleterious effects on the transplanted kidney function or on episodes of graft rejection. The motivation for this type of study is:
Effective vaccines have been developed against a number of deadly diseases, including viral diseases such as measles, mumps, polio, and smallpox, as well as several bacterial infections. However, there remain several important infectious diseases for which vaccines are currently lacking. One important aspect of developing effective vaccines against these pathogens is:
Type 1 diabetes has traditionally been considered a T-cell-mediated autoimmune disease. However, recent studies have shown that treatment of newly diagnosed type 1 diabetes patients with rituximab (ant-CD20 antibody) had a significant effect in slowing down the progression of their disease. These results indicate that autoantibodies likely contribute to the process of pancreatic -islet cell destruction in these patients.
The first JAK kinase inhibitor to be developed, tofacitinib, inhibits JAK1 and JAK3. These two JAK kinases are required for the signaling pathways induced by multiple cytokines, including all of the receptors that share the cytokine receptor common gamma chain ( c; includes the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21), all of the receptors that share gp130 (includes the receptor for IL-6), the receptors for GM-CSF, IL-12, and IL-23, and both type I and type II (i.e., IFN- ) interferon receptors. Tofacitinib has recently been shown to be effective in the treatment of severe rheumatoid arthritis, an autoimmune disease characterized by inflammation of the joints with prominent inflammatory cell infiltrates, autoantibodies, and eventual cartilage and bone destruction. A likely explanation for the therapeutic benefits of tofacitinib in this disease is:
Abatacept is a biologic drug that is a fusion protein formed from the B7-binding domain of CTLA-4 fused to the human IgG1 constant region. In patients suffering from rheumatoid arthritis (RA), high levels of granzyme B in the synovial fluid of inflamed joints is thought to contribute to joint erosion. When treated with abatacept, a subset of patients shows significant improvement in their RA symptoms together with decreased levels of granzyme B in their affected joints. These data indicate that the granzyme B found in RA patients' inflamed joints is predominantly made by:
One early study on DNA vaccines examined the CD8 T cell response to the plasmid-encoded antigenic protein following subcutaneous immunization of mice with the plasmid DNA. This study used bone marrow chimeras to distinguish the MHC class I alleles expressed on bone marrow-derived cells from those expressed on all other mouse cells and tissues. When analyzed, the data showed that antigen-specific CD8 T cell responses were restricted to the MHC class I alleles derived from the donor bone marrow. These findings indicate that a key step in DNA vaccination is:
Immune responses to tumors have been studied extensively in mice, using transplantable tumors injected into syngeneic mice. The basis for many of these studies is the assumption that the process of tumorigenesis generates mutations in genes encoding self-antigens that would allow the immune system to see these mutant proteins as 'foreign'. In this scenario, the dominant immune response targeting the tumor cells would be mediated by:
One of the first biologics developed was a drug known as etanercept (common name, Enbrel) that is a fusion protein formed from the cytokine binding domain of the TNF-receptor fused to the human IgG1 constant region, as shown in Figure Q6). This biologic is used to treat patients with inflammatory diseases such as rheumatoid arthritis, psoriasis, and others. Etanercept was developed to avoid injecting patients with the mouse monoclonal antibody to TNF- , which was available at the time. 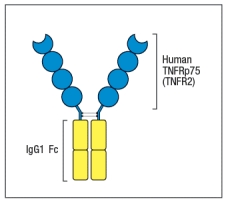 Figure Q6) Etanercept was preferred because:
Figure Q6) Etanercept was preferred because:
Cyclosporin A and rapamycin are each used as T cell immunosuppressants. They share the property of binding to immunophilin molecules in T cells as the initial step in their mechanisms of action. However, in the case of cyclosporin A, the drug:immunophilin complex binds to and inhibits the protein phosphatase calcineurin, whereas the rapamycin:immunophilin complex binds to and inhibitors mTOR. As a consequence,
One clinical trial performed in the early 2000s tested whether addition of unmethylated CpG oligonucleotides (CpG ODN) to the hepatitis B vaccine improved immune responses in volunteer vaccinees. The normal vaccine consists of purified recombinant hepatitis B surface antigen protein mixed with alum. Half of the subjects received this original vaccine, and half received the original vaccine with added CpG ODN. The study found that individuals receiving the vaccine plus CpG ODN showed more rapid and robust antibody responses, and a trend toward enhanced CD8 cytotoxic T cell responses compared to the vaccine alone group. As expected, the group receiving the vaccine plus CpG ODN also:
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)