Deck 8: Development and Survival of Lymphocytes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 8: Development and Survival of Lymphocytes
1
B cell development in the bone marrow is an inherently wasteful process. Nearly half of the pro-B cells produced will die without progressing on to the next stage of B cell development. This massive loss of pro-B cells is due to:
A) The failure of many pro-B cells to up-regulate Pax5 and become committed to the B cell lineage.
B) The inability of many pro-B cells to proceed with rearranging a VH to their rearranged DJH sequence.
C) Large insertions of untemplated nucleotides into the rearranged gene by TdT.
D) Detrimental DJH rearrangements on both alleles of the immunoglobulin heavy chain locus.
E) The failure of the pro-B cell to make a complete immunoglobulin heavy chain protein.
A) The failure of many pro-B cells to up-regulate Pax5 and become committed to the B cell lineage.
B) The inability of many pro-B cells to proceed with rearranging a VH to their rearranged DJH sequence.
C) Large insertions of untemplated nucleotides into the rearranged gene by TdT.
D) Detrimental DJH rearrangements on both alleles of the immunoglobulin heavy chain locus.
E) The failure of the pro-B cell to make a complete immunoglobulin heavy chain protein.
The failure of the pro-B cell to make a complete immunoglobulin heavy chain protein.
2
A key step in the development of B cells is the expression of the RAG-1 and RAG-2 recombinase proteins. The up-regulation of RAG-1 and RAG-2 in early pro-B cells is induced by:
A) The cytokine IL-7, which is made by bone marrow stromal cells
B) The chemokine CXCL12, which is made by bone marrow stromal cells
C) The B cell-specific transcription factors E2A and EBF
D) Signaling through the Igα subunit of the B-cell receptor complex
E) Signaling through the FLT3 receptor tyrosine kinase binding to membrane-bound FLT3
A) The cytokine IL-7, which is made by bone marrow stromal cells
B) The chemokine CXCL12, which is made by bone marrow stromal cells
C) The B cell-specific transcription factors E2A and EBF
D) Signaling through the Igα subunit of the B-cell receptor complex
E) Signaling through the FLT3 receptor tyrosine kinase binding to membrane-bound FLT3
The B cell-specific transcription factors E2A and EBF
3
B and T lymphocytes develop from multipotent hematopoietic stem cells in the bone marrow. This process entails a continuum of development in which cells show progressive loss of multipotent potential, eventually becoming committed to a single lineage.
True
4
Two distinct lineages of T cells can be identified based on their expression of : versus : T-cell receptors. A deficiency in the signaling receptor Notch1 would result in:
A) A loss of : but not : T cells
B) A loss of both of : and : T cells
C) A loss of : T cells and a massive expansion of : T cells
D) An increased number of both of : and : T cells
E) A normal number of both of : and : T cells
A) A loss of : but not : T cells
B) A loss of both of : and : T cells
C) A loss of : T cells and a massive expansion of : T cells
D) An increased number of both of : and : T cells
E) A normal number of both of : and : T cells

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
In different mammalian species, the ratio of B cells expressing versus light chain-containing antibodies is about 65%:35%. In other species, such as mice, this ratio is vastly different, at 95%:5%. If a routine blood test performed on an individual revealed that their -expressing versus -expressing B cells were seen at a ratio of 95%:5%, this would likely indicate that the individual had:
A) An increased number of functional V gene segments compared to the average human in the population
B) A defect in allelic exclusion of antibody light chain genes
C) A defect in isotypic exclusion of antibody light chains
D) A lymphoproliferative disorder
E) A genetic defect in one of their two light chain alleles
A) An increased number of functional V gene segments compared to the average human in the population
B) A defect in allelic exclusion of antibody light chain genes
C) A defect in isotypic exclusion of antibody light chains
D) A lymphoproliferative disorder
E) A genetic defect in one of their two light chain alleles

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Self-reactive B cells can be eliminated from the repertoire at several stages of B cell maturation, including immature B cells that have already emigrated from the bone marrow into the circulation. This latter stage of tolerance induction is critical because:
A) Not all self-antigens are expressed or present in the bone marrow during B cell development.
B) Immature circulating B cells are more sensitive to antigen stimulation than the developing B cells in the bone marrow.
C) Receptor editing is not a perfect process and some self-reactive B cells may fail to be eliminated in the bone marrow.
D) Circulating immature B cells do not encounter tissue-specific antigens in peripheral organs and tissues.
E) Immature B cells are trapped in the bone marrow by strong B-cell receptor cross-linking.
A) Not all self-antigens are expressed or present in the bone marrow during B cell development.
B) Immature circulating B cells are more sensitive to antigen stimulation than the developing B cells in the bone marrow.
C) Receptor editing is not a perfect process and some self-reactive B cells may fail to be eliminated in the bone marrow.
D) Circulating immature B cells do not encounter tissue-specific antigens in peripheral organs and tissues.
E) Immature B cells are trapped in the bone marrow by strong B-cell receptor cross-linking.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
Marginal zone B cells are thought to represent a lineage of cells important in rapid responses to blood-borne antigens. What are the two characteristics of these cells that indicate this function?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
B-1 B cells are considered a component of the innate rather than the adaptive immune response. The antibodies produced by B-1 B cells generally recognize capsular polysaccharide antigens found on many bacteria and viruses. These antibodies are considered part of the innate immune response because:
A) They recognize pathogens rather than innocuous harmless antigens.
B) They are produced prior to the exposure to the pathogen.
C) They are specific for carbohydrate rather than protein antigens.
D) They are secreted by B-1 B cells starting at 48 hour post-infection.
E) They are not generated by the process of VD-J recombination of immunoglobulin genes.
A) They recognize pathogens rather than innocuous harmless antigens.
B) They are produced prior to the exposure to the pathogen.
C) They are specific for carbohydrate rather than protein antigens.
D) They are secreted by B-1 B cells starting at 48 hour post-infection.
E) They are not generated by the process of VD-J recombination of immunoglobulin genes.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are characterized by high levels of circulating autoreactive antibodies in the patient's circulation. An analysis of developing B cells in the bone marrow of these individuals might reveal that in some cases:
A) Developing B cells become anergic in response to strong B-cell receptor cross-linking by multivalent self-antigens.
B) Developing B cells are excluded from the B-cell follicles in the bone marrow and undergo rapid cell death.
C) sIgM-positive developing B cells show premature down-regulation of RAG recombinase proteins prior to the onset of receptor editing.
D) Hyperactive B-cell receptor signaling leads to rapid turnover of self-reactive developing B cells.
E) Developing B cells have defects in allelic exclusion and express two different B-cell receptors on their surface.
A) Developing B cells become anergic in response to strong B-cell receptor cross-linking by multivalent self-antigens.
B) Developing B cells are excluded from the B-cell follicles in the bone marrow and undergo rapid cell death.
C) sIgM-positive developing B cells show premature down-regulation of RAG recombinase proteins prior to the onset of receptor editing.
D) Hyperactive B-cell receptor signaling leads to rapid turnover of self-reactive developing B cells.
E) Developing B cells have defects in allelic exclusion and express two different B-cell receptors on their surface.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
Unlike : T cells, : T cells are considered to be components of the innate immune system. One feature of : T cells that leads to their classification as innate cells is:
A) That they migrate from the thymus directly to barrier surfaces such as mucosa and epithelia
B) Their ability to produce pro-inflammatory cytokines
C) That their T-cell receptors are germline encoded rather than a product of VD-J recombination
D) Their rapid turnover in the tissue, with an average survival time of 3-4 days
E) Their ability to make growth factors that act on epithelial cells rather than other hematopoietic cells
A) That they migrate from the thymus directly to barrier surfaces such as mucosa and epithelia
B) Their ability to produce pro-inflammatory cytokines
C) That their T-cell receptors are germline encoded rather than a product of VD-J recombination
D) Their rapid turnover in the tissue, with an average survival time of 3-4 days
E) Their ability to make growth factors that act on epithelial cells rather than other hematopoietic cells

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Immature B cells expressing sIgM receptor emigrate from the bone marrow into the circulation. This is a passive process of cell diffusion, requiring no active signaling by the B cell.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
The pre-B-cell receptor provides an important signal that induces transition of pro-B cells to pre-B cells. An important characteristic of this receptor is that:
A) It signals without binding to an extracellular ligand.
B) It is composed of immunoglobulin heavy chains and the VJ region of a rearranged light chain.
C) It is expressed at very high levels on the surface of the pro-B cell.
D) It signals without requiring association with B-cell receptor signaling subunits, Ig and Ig .
E) It signals without requiring the B-cell receptor signaling kinase, BTK.
A) It signals without binding to an extracellular ligand.
B) It is composed of immunoglobulin heavy chains and the VJ region of a rearranged light chain.
C) It is expressed at very high levels on the surface of the pro-B cell.
D) It signals without requiring association with B-cell receptor signaling subunits, Ig and Ig .
E) It signals without requiring the B-cell receptor signaling kinase, BTK.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
The mouse thymus normally contains about 1–2 x 108 thymocytes, the vast majority of which are CD4+CD8+ (double-positive) cells. When thymocytes from mice with a gene deficiency in the TCR locus are compared with those from TCR -deficient mice, a striking difference between the two different knockout lines is observed, as shown in Figure in a simplified version of flow cytometry data. The numbers of thymocytes in each thymus is indicated below the plots.
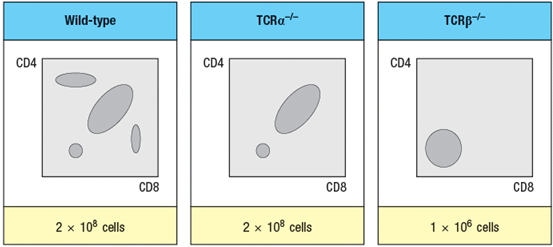
-Which region of the thymus organ would show a dearth of developing thymocytes in the TCR -/- thymus? Which region in the TCR -/- thymus?

-Which region of the thymus organ would show a dearth of developing thymocytes in the TCR -/- thymus? Which region in the TCR -/- thymus?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Progenitor cells that migrate from the bone marrow to the thymus are not yet committed to the T cell lineage. T cell lineage commitment occurs as a result of signals received by the progenitor cell from thymic epithelial cells.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
A wild-type mouse that is heterozygous for two immunoglobulin heavy chain alleles (IgHa/b) generates the population of B cells shown on the left of Figure . A mouse strain, also IgHa/b, carries an inactivating mutation in the VpreB gene. In addition to producing fewer mature B cells than the wild-type mice, the VpreB-deficient mice generate B cells as shown on the right. 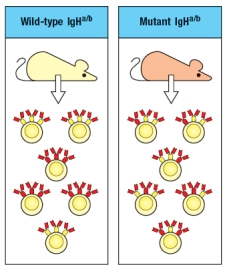
What is the explanation of the difference seen between the wild-type and the VpreB-mutant B cells?

What is the explanation of the difference seen between the wild-type and the VpreB-mutant B cells?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
The mouse thymus normally contains about 1–2 x 108 thymocytes, the vast majority of which are CD4+CD8+ (double-positive) cells. When thymocytes from mice with a gene deficiency in the TCR locus are compared with those from TCR -deficient mice, a striking difference between the two different knockout lines is observed, as shown in Figure in a simplified version of flow cytometry data. The numbers of thymocytes in each thymus is indicated below the plots.

-What is the explanation for the difference in thymocyte subsets and cell numbers observed when comparing TCR -/- to TCR -/- thymocytes?

-What is the explanation for the difference in thymocyte subsets and cell numbers observed when comparing TCR -/- to TCR -/- thymocytes?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Individuals that overexpress the cytokine BAFF show increased susceptibility to autoimmune diseases such as Sjögren's syndrome, a disease that targets the exocrine glands that produce saliva, tears, and other bodily secretions. If one examined the circulating antibodies in these patients, one would expect to find:
A) Increased development of B cells in the bone marrow
B) A failure of receptor editing of immunoglobulin light chain genes in the bone marrow
C) An increased rate of immature B cell export from the bone marrow
D) Reduced B-cell receptor signaling following strong cross-linking of the receptor
E) An increased number of circulating mature autoreactive B cells
A) Increased development of B cells in the bone marrow
B) A failure of receptor editing of immunoglobulin light chain genes in the bone marrow
C) An increased rate of immature B cell export from the bone marrow
D) Reduced B-cell receptor signaling following strong cross-linking of the receptor
E) An increased number of circulating mature autoreactive B cells

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Genetically inherited immunodeficiency diseases can result from defects in nearly any component of the immune response. The most severe forms of immunodeficiency occur when T cells are absent or non-functional. An individual with normal B cells, but an absence of T cells might have a defect in:
A) RAG-1 or RAG-2 recombinase proteins
B) Terminal deoxynucleotidyl transferase (TdT)
C) Hematopoietic stem cells
D) Bone marrow stromal cells
E) Thymic stromal cells
A) RAG-1 or RAG-2 recombinase proteins
B) Terminal deoxynucleotidyl transferase (TdT)
C) Hematopoietic stem cells
D) Bone marrow stromal cells
E) Thymic stromal cells

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
The thymic cortex has a substantial population of macrophages in addition to the developing T cells (i.e., thymocytes). These macrophages are extremely useful in:
A) Eliminating bacterial infections in the thymus
B) Producing cytokines that promote T cell maturation
C) Engulfing apoptotic thymocytes
D) Maintaining the structural integrity of the thymic organ
E) Inducing inflammatory signals to increase blood flow to the thymus
A) Eliminating bacterial infections in the thymus
B) Producing cytokines that promote T cell maturation
C) Engulfing apoptotic thymocytes
D) Maintaining the structural integrity of the thymic organ
E) Inducing inflammatory signals to increase blood flow to the thymus

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
One feature of : T cells that identifies them as innate, rather than adaptive, lymphocytes is their ability to produce effector cytokines within hours of initial activation.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
Two mutant lines of mice have been identified, each of which has a defect in T cell development. The subsets of thymocytes found in each mutant mouse line are shown in Figure
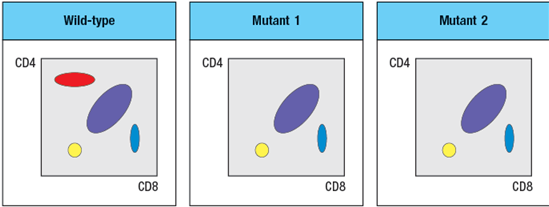
To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:
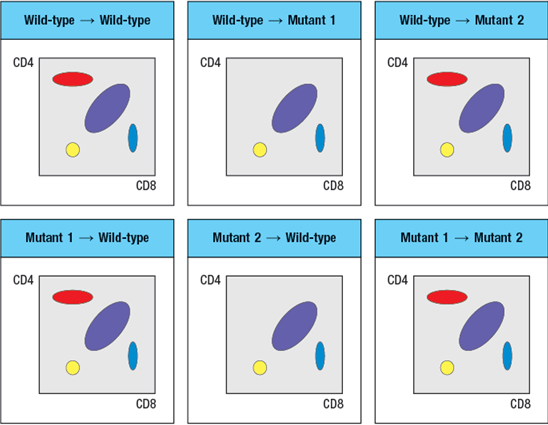
A potential candidate molecule for the gene that is defective in Mutant-1 is:
A) CD4
B) MHC class II
C) Th-POK
D) MHC class I
E) Runx3

To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:

A potential candidate molecule for the gene that is defective in Mutant-1 is:
A) CD4
B) MHC class II
C) Th-POK
D) MHC class I
E) Runx3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Proteins found in the circulation travel throughout the body, including the thymus. One example is serum albumin. Developing T cells with T-cell receptors specific for peptides of human serum albumin bound to MHC class II molecules would likely be:
A) Positively selected and would mature into CD4 T cells
B) Positively selected and would mature into CD8 T cells
C) Negatively selected in the thymus and deleted from the mature repertoire
D) Targeted for peripheral mechanisms of self-tolerance after emigrating from the thymus
E) Excluded from the T cell zones of the spleen after emigrating from the thymus
A) Positively selected and would mature into CD4 T cells
B) Positively selected and would mature into CD8 T cells
C) Negatively selected in the thymus and deleted from the mature repertoire
D) Targeted for peripheral mechanisms of self-tolerance after emigrating from the thymus
E) Excluded from the T cell zones of the spleen after emigrating from the thymus

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
Some specialized subsets of : T cells complete their development in the thymus and avoid negative selection, in spite of having T-cell receptors with high affinity for self-MHC complexes.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
The repertoire of T-cell receptors, like that of antibodies, is formed by the random rearrangement of multiple gene segments that combine to generate the variable domain of each receptor subunit. The bias of T-cell receptors for binding to peptide:MHC complexes, rather than to all possible antigenic structures like antibodies, is simply the result of positive selection in the thymus.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
Experimental mouse models have been developed to study the mechanisms leading to the breakdown of self-tolerance and the onset of autoimmunity. One strategy is to express a foreign antigen, such as a viral protein, in a single defined cell type in a peripheral organ. For instance, the lymphocytic choriomeningitis virus (LCMV) glycoprotein has been expressed in β-islet cells of the pancreas by making a line of mice that is transgenic for a construct linking the LCMV-glycoprotein gene to the insulin promoter. In these transgenic mice, the LCMV protein is expressed only in pancreatic -islet cells. Thymocytes with T-cell receptors specific for a peptide of LCMV-glycoprotein bound to MHC class I develop normally in the thymus, and do not undergo negative selection. The fate of these T cells once they emigrate from the thymus would likely be:
A) They would be activated in the periphery and attach and kill the pancreatic -islet cells.
B) They would either be deleted in the periphery or would become unresponsive.
C) They would induce an inflammatory response in the pancreas that would up-regulate co-stimulatory molecules on antigen-presenting cells.
D) They would secrete cytokines that promote T cell proliferation.
E) They would differentiate into virus-specific memory T cells that would protect mice upon infection with LCMV.
A) They would be activated in the periphery and attach and kill the pancreatic -islet cells.
B) They would either be deleted in the periphery or would become unresponsive.
C) They would induce an inflammatory response in the pancreas that would up-regulate co-stimulatory molecules on antigen-presenting cells.
D) They would secrete cytokines that promote T cell proliferation.
E) They would differentiate into virus-specific memory T cells that would protect mice upon infection with LCMV.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Synthesis question: To investigate how T-cell receptor signaling regulates T cell development in the thymus, a mutant mouse is generated with a deficiency in the T cell tyrosine kinase, LCK (Lck-/- mice). Analysis of thymocytes from these mice, stained with antibodies to CD4 and CD8, is shown in Figure.
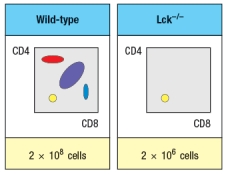
a) What is the explanation for the altered number and subsets of thymocytes in Lck-/- mice?
Due to the defect observed in the germline Lck-deficient mice, it is not possible to use these mice to examine any potential role for this T-cell receptor signaling protein at later stages of thymocyte development. To circumvent this problem, conditional Lck-deficient mice are generated by crossing mice with a homozygous 'floxed' allele of Lck (Lckfl/fl) to mice that express the cre recombinase in CD4+CD8+ double-positive thymocytes (i.e., CD4-cre). When cre is expressed at the double-positive stage, the Lck gene is deleted, and thymocytes become Lck-deficient at that time. The thymocyte profile of these Lckfl/fl x CD4-cre mice is shown in Figure.
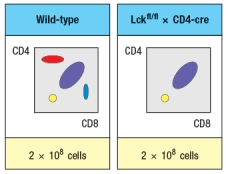
b) What is the explanation for the altered thymocyte profile seen in Lckfl/fl x CD4-cre mice?
To further assess the impact of altered T-cell receptor signaling on thymocyte development, another line of mice is generated that express a super-active version of the Lck kinase (called 'Lck-super') under control of the CD4 promoter. This super-active Lck is expressed starting at the double-positive stage in the thymus. Lck-super is activated by T-cell receptor signaling, just like wild-type Lck, but when activated has an approximately tenfold increased kinase activity. Surprisingly, mice expressing Lck-super do not develop increased numbers of mature T cells, but instead, show the following.
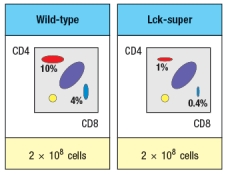
c) What is the most likely explanation for the altered profile of thymocytes seen in the Lck-super mice?
Struck by the findings in the Lck-super mice, an investigator performs one further experiment. This researcher clones the rearranged T-cell receptor and chain genes from two CD4 single-positive thymocytes: one that is isolated from a WT thymus, and the second, isolated from an Lck-super thymus. Each T-cell receptor : pair is used to generate a T-cell receptor transgenic line, so that nearly 100% of all double-positive thymocytes in each transgenic line express only the transgenic T-cell receptor. The two T cell receptor transgenics are named based on which mouse line the receptor chains were originally isolated from. The one from the wild-type line is known as TCR-tgwt, and the one originally isolated from the Lck-super line is known as TCR-tgsuper. In each case, thymocytes from the T-cell receptor transgenic lines are analyzed on a wild-type background, or after crossing to the Lck-super line. The results are shown in Figure.
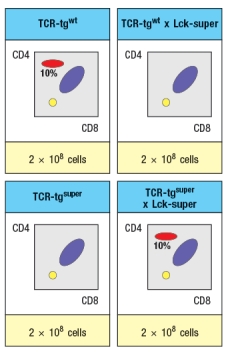
d) Explain the results observed in this experiment.

a) What is the explanation for the altered number and subsets of thymocytes in Lck-/- mice?
Due to the defect observed in the germline Lck-deficient mice, it is not possible to use these mice to examine any potential role for this T-cell receptor signaling protein at later stages of thymocyte development. To circumvent this problem, conditional Lck-deficient mice are generated by crossing mice with a homozygous 'floxed' allele of Lck (Lckfl/fl) to mice that express the cre recombinase in CD4+CD8+ double-positive thymocytes (i.e., CD4-cre). When cre is expressed at the double-positive stage, the Lck gene is deleted, and thymocytes become Lck-deficient at that time. The thymocyte profile of these Lckfl/fl x CD4-cre mice is shown in Figure.

b) What is the explanation for the altered thymocyte profile seen in Lckfl/fl x CD4-cre mice?
To further assess the impact of altered T-cell receptor signaling on thymocyte development, another line of mice is generated that express a super-active version of the Lck kinase (called 'Lck-super') under control of the CD4 promoter. This super-active Lck is expressed starting at the double-positive stage in the thymus. Lck-super is activated by T-cell receptor signaling, just like wild-type Lck, but when activated has an approximately tenfold increased kinase activity. Surprisingly, mice expressing Lck-super do not develop increased numbers of mature T cells, but instead, show the following.

c) What is the most likely explanation for the altered profile of thymocytes seen in the Lck-super mice?
Struck by the findings in the Lck-super mice, an investigator performs one further experiment. This researcher clones the rearranged T-cell receptor and chain genes from two CD4 single-positive thymocytes: one that is isolated from a WT thymus, and the second, isolated from an Lck-super thymus. Each T-cell receptor : pair is used to generate a T-cell receptor transgenic line, so that nearly 100% of all double-positive thymocytes in each transgenic line express only the transgenic T-cell receptor. The two T cell receptor transgenics are named based on which mouse line the receptor chains were originally isolated from. The one from the wild-type line is known as TCR-tgwt, and the one originally isolated from the Lck-super line is known as TCR-tgsuper. In each case, thymocytes from the T-cell receptor transgenic lines are analyzed on a wild-type background, or after crossing to the Lck-super line. The results are shown in Figure.

d) Explain the results observed in this experiment.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
If one swapped the regulatory elements of the TCR and TCR loci, so that rearrangement and expression of the TCR locus occurred first, in double-negative thymocytes, and TCR rearrangement and expression were delayed until the double-positive stage, would T cell development proceed normally to generate mature T cells? Why or why not?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
The final stages of T cell development occur in the thymic medulla, after the developing cells become CD4 or CD8 single-positive. One important change that occurs during this final maturation is:
A) The down-regulation of the pre-T-cell receptor (pre-T ) chain protein
B) The up-regulation of genes encoding effector cytokines and cytolytic effector proteins
C) The increased susceptibility to T-cell receptor-induced apoptosis
D) The loss of susceptibility to T-cell receptor-induced apoptosis
E) The up-regulation of signaling proteins required for T cell activation
A) The down-regulation of the pre-T-cell receptor (pre-T ) chain protein
B) The up-regulation of genes encoding effector cytokines and cytolytic effector proteins
C) The increased susceptibility to T-cell receptor-induced apoptosis
D) The loss of susceptibility to T-cell receptor-induced apoptosis
E) The up-regulation of signaling proteins required for T cell activation

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
An infant is admitted to the hospital with a history of recurrent and persistent bacterial infections. His physician suspects he has an immunodeficiency disease, and obtains a sample of the patient's peripheral blood. The white blood cells are analyzed by antibody staining followed by flow cytometry, and the results are shown in Figure.
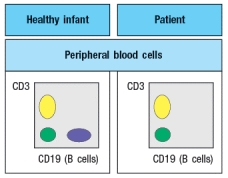
To determine the origin of the peripheral blood cell defect, a bone marrow biopsy is taken from the patient and compared to a healthy control, as shown in Figure.
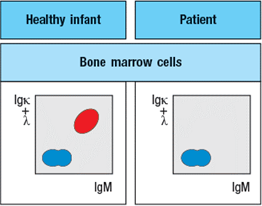
To obtain additional information, bone marrow cells are treated with a chemical that permeabilizes the cell membrane, allowing antibodies to enter the cells and bind to their target antigens within the cells, a technique known as ‘intracellular staining’. The results of this analysis are shown in Figure .
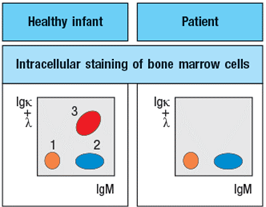
a) What are populations 1, 2, and 3 in Figure?
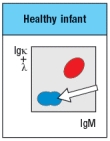
b) In the analysis of cell surface expression (non-permeabilized bone marrow cells), what are the IgMlo Igκ+λneg cells as indicated by the arrow in Figure?
c) What is the most likely defect causing the patient's immunodeficiency?
The results shown above indicate a specific defect in B cell development. To help identify the defective or missing protein in the patient's developing B cells, bone marrow cells are isolated and protein lysates are prepared for immunoblotting. A series of antibodies are tested and the results are shown in Figure.
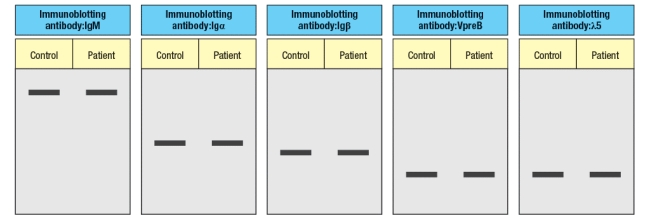
d) Given the results from the immunoblotting experiments, what is the most likely candidate molecule (or type of molecule) responsible for the patient's immunodeficiency disease?

To determine the origin of the peripheral blood cell defect, a bone marrow biopsy is taken from the patient and compared to a healthy control, as shown in Figure.

To obtain additional information, bone marrow cells are treated with a chemical that permeabilizes the cell membrane, allowing antibodies to enter the cells and bind to their target antigens within the cells, a technique known as ‘intracellular staining’. The results of this analysis are shown in Figure .

a) What are populations 1, 2, and 3 in Figure?
b) In the analysis of cell surface expression (non-permeabilized bone marrow cells), what are the IgMlo Igκ+λneg cells as indicated by the arrow in Figure?

c) What is the most likely defect causing the patient's immunodeficiency?
The results shown above indicate a specific defect in B cell development. To help identify the defective or missing protein in the patient's developing B cells, bone marrow cells are isolated and protein lysates are prepared for immunoblotting. A series of antibodies are tested and the results are shown in Figure.

d) Given the results from the immunoblotting experiments, what is the most likely candidate molecule (or type of molecule) responsible for the patient's immunodeficiency disease?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
MHC class II molecules expressed on the surface of thymic cortical epithelial cells normally have a wide repertoire of different peptides bound to them. By engineering a construct that fuses the MHC class II protein to a single peptide sequence, and expressing this construct in thymic cortical epithelial cells that have their endogenous MHC class II genes knocked out, it is possible to generate a mouse line where all MHC class II proteins expressed on all thymic cortical epithelial cells are bound to the same peptide. These mice are often referred to as 'single-peptide' mice. Examination of the T cell developing in these single peptide mice would likely show:
A) A significant reduction in the numbers of mature CD4 T cells
B) No change in the numbers of mature CD4 T cells
C) A block in T cell development at the CD4+CD8+ double-positive stage
D) A repertoire of T-cell receptors on mature CD4 T cells restricted to a single V
E) A block in T cell development at the CD4-CD8- double-negative stage
A) A significant reduction in the numbers of mature CD4 T cells
B) No change in the numbers of mature CD4 T cells
C) A block in T cell development at the CD4+CD8+ double-positive stage
D) A repertoire of T-cell receptors on mature CD4 T cells restricted to a single V
E) A block in T cell development at the CD4-CD8- double-negative stage

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
Current evidence indicates that >95% of the CD4+CD8+ double-positive thymocytes generated will die in the thymus, and will never develop into mature CD4 or CD8 T cells. While a small proportion of these double-positive thymocytes may fail to produce a functional : T-cell receptor, the majority of them do express a T-cell receptor complex on their surface. For any given double-positive thymocyte undergoing cell death in the thymus, what are the two possible explanations for its failure to mature?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
Like the : T cells in other specific mucosal surfaces, the : T cells that reside in the epithelial surface of the skin:
A) Are slow to respond to activation signals, requiring several days of priming and differentiation
B) Are able to produce cytokines that simultaneously induce type I, type II, and type III immune responses
C) Are primarily responsible for recruiting macrophages and dendritic cells to the tissue
D) Are characterized by the homogeneous expression of a single specific V and V in their T-cell receptors
E) Are unlikely to play any role in immunity to infection, but are likely important for tissue repair
A) Are slow to respond to activation signals, requiring several days of priming and differentiation
B) Are able to produce cytokines that simultaneously induce type I, type II, and type III immune responses
C) Are primarily responsible for recruiting macrophages and dendritic cells to the tissue
D) Are characterized by the homogeneous expression of a single specific V and V in their T-cell receptors
E) Are unlikely to play any role in immunity to infection, but are likely important for tissue repair

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
Two mutant lines of mice have been identified, each of which has a defect in T cell development. The subsets of thymocytes found in each mutant mouse line are shown in Figure

To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:

A potential candidate molecule for the gene that is defective in Mutant-2 is:
A) CD4
B) MHC class II
C) Th-POK
D) MHC class I
E) Runx3

To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:

A potential candidate molecule for the gene that is defective in Mutant-2 is:
A) CD4
B) MHC class II
C) Th-POK
D) MHC class I
E) Runx3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
Approximately one in every three : T cells expresses two different rearranged TCR chain proteins. Yet T cells are still considered to have 'clonal specificity' for recognizing antigen. The reason for asserting that each T cell has a single functional specificity for recognizing antigen is that:
A) Only one of the two TCR chains expressed by a T cell will pair with its TCR chain.
B) T cells expressing two different TCR chains will die by apoptosis when they are activated.
C) Only one T-cell receptor expressed by each T cell will recognize peptide presented by self-MHC molecules.
D) The majority of T cells in an individual will never encounter their specific peptide-MHC ligand and so will not be part of an immune response.
E) The majority of T cells are self-reactive and therefore eliminated during their development in the thymus.
A) Only one of the two TCR chains expressed by a T cell will pair with its TCR chain.
B) T cells expressing two different TCR chains will die by apoptosis when they are activated.
C) Only one T-cell receptor expressed by each T cell will recognize peptide presented by self-MHC molecules.
D) The majority of T cells in an individual will never encounter their specific peptide-MHC ligand and so will not be part of an immune response.
E) The majority of T cells are self-reactive and therefore eliminated during their development in the thymus.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Experiments performed with T-cell receptor transgenic mice identified the fate of developing thymocytes that failed positive selection. Based on these findings, examination of thymocytes in MHC class I-MHC class II-deficient mice (lacking all MHC class I and class II expression in the thymus) would show:
A) A 100-fold decrease in total thymocytes numbers
B) A block in T cell development at the CD4-CD8- double-negative stage
C) Normal numbers and subsets of thymocytes and peripheral T cells
D) Normal numbers of thymocytes, but no peripheral T cells
E) A block in T cell development at the CD4+CD8+ double-positive stage
A) A 100-fold decrease in total thymocytes numbers
B) A block in T cell development at the CD4-CD8- double-negative stage
C) Normal numbers and subsets of thymocytes and peripheral T cells
D) Normal numbers of thymocytes, but no peripheral T cells
E) A block in T cell development at the CD4+CD8+ double-positive stage

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
While many cell types in the thymus are able to induce negative selection of developing self-reactive thymocytes, bone marrow-derived antigen-presenting cells, such as macrophages and dendritic cells, appear to be the most important for this process. One likely reason for the prominent role of bone marrow-derived antigen-presenting cells in inducing negative selection of developing thymocytes is:
A) Bone marrow-derived antigen-presenting cells are the most abundant stromal cells in the thymus.
B) Bone marrow-derived antigen-presenting cells are very good at inducing mature T cell activation.
C) Bone marrow-derived antigen-presenting cells are highly phagocytic and have specialized mechanisms for presenting peptides on both MHC class I and class II.
D) Bone marrow-derived antigen-presenting cells are concentrated in the thymic medulla where negative selection is most prominent.
E) Bone marrow-derived antigen-presenting cells are hematopoietic in origin, so share the same genetic make-up as the developing thymocytes.
A) Bone marrow-derived antigen-presenting cells are the most abundant stromal cells in the thymus.
B) Bone marrow-derived antigen-presenting cells are very good at inducing mature T cell activation.
C) Bone marrow-derived antigen-presenting cells are highly phagocytic and have specialized mechanisms for presenting peptides on both MHC class I and class II.
D) Bone marrow-derived antigen-presenting cells are concentrated in the thymic medulla where negative selection is most prominent.
E) Bone marrow-derived antigen-presenting cells are hematopoietic in origin, so share the same genetic make-up as the developing thymocytes.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
T cell development in the thymus shares some similarities to a pipeline. As new progenitor cells enter the thymus, the most mature thymocytes are pushed out of the thymus to enter the circulation by a passive process.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck


