Exam 8: Development and Survival of Lymphocytes
Exam 1: Basic Concepts in Immunology44 Questions
Exam 2: Innate Immunity: the First Lines of Defense32 Questions
Exam 3: The Induced Responses of Innate Immunity39 Questions
Exam 4: Antigen Recognition by B-Cell and T-Cell Receptors28 Questions
Exam 5: The Generation of Lymphocyte Antigen Receptors33 Questions
Exam 6: Antigen Presentation to T Lymphocytes30 Questions
Exam 7: Lymphocyte Receptor Signaling42 Questions
Exam 8: Development and Survival of Lymphocytes37 Questions
Exam 9: T-Cell-Mediated Immunity37 Questions
Exam 10: The Humoral Immune Response30 Questions
Exam 11: Integrated Dynamics of Innate and Adaptive Immunity28 Questions
Exam 12: The Mucosal Immune System27 Questions
Exam 13: Failures of Host Defense Mechanisms43 Questions
Exam 14: Allergy and Allergic Diseases26 Questions
Exam 15: Autoimmunity and Transplantation31 Questions
Exam 16: Manipulation of the Immune Response34 Questions
Select questions type
MHC class II molecules expressed on the surface of thymic cortical epithelial cells normally have a wide repertoire of different peptides bound to them. By engineering a construct that fuses the MHC class II protein to a single peptide sequence, and expressing this construct in thymic cortical epithelial cells that have their endogenous MHC class II genes knocked out, it is possible to generate a mouse line where all MHC class II proteins expressed on all thymic cortical epithelial cells are bound to the same peptide. These mice are often referred to as 'single-peptide' mice. Examination of the T cell developing in these single peptide mice would likely show:
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
The mouse thymus normally contains about 1–2 x 108 thymocytes, the vast majority of which are CD4+CD8+ (double-positive) cells. When thymocytes from mice with a gene deficiency in the TCR locus are compared with those from TCR -deficient mice, a striking difference between the two different knockout lines is observed, as shown in Figure in a simplified version of flow cytometry data. The numbers of thymocytes in each thymus is indicated below the plots.
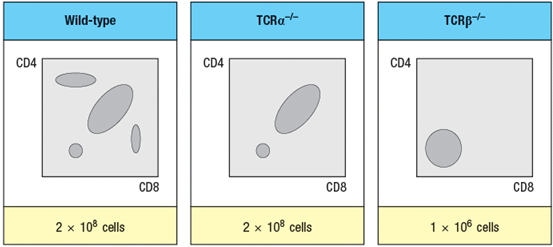 -What is the explanation for the difference in thymocyte subsets and cell numbers observed when comparing TCR -/- to TCR -/- thymocytes?
-What is the explanation for the difference in thymocyte subsets and cell numbers observed when comparing TCR -/- to TCR -/- thymocytes?
Free
(Essay)
4.9/5  (28)
(28)
Correct Answer:
The TCR chain, but not the TCR chain is required for the pre-TCR to assemble at the CD4-CD8- (double-negative) stage of thymocyte development. The pre-TCR signal is critical for inducing double-negative thymocytes to differentiate into CD4+CD8+ double-positive thymocytes. This signal is also essential to induce multiple rounds of cell division as thymocytes progress from double-negative to double-positive. This proliferation leads to ~100-fold increase in thymocyte numbers. As a consequence, double-negative thymocytes lacking TCR will fail to proliferate and will fail to differentiate into double-positive cells.
Two distinct lineages of T cells can be identified based on their expression of : versus : T-cell receptors. A deficiency in the signaling receptor Notch1 would result in:
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
B
Like the : T cells in other specific mucosal surfaces, the : T cells that reside in the epithelial surface of the skin:
(Multiple Choice)
4.8/5  (33)
(33)
Approximately one in every three : T cells expresses two different rearranged TCR chain proteins. Yet T cells are still considered to have 'clonal specificity' for recognizing antigen. The reason for asserting that each T cell has a single functional specificity for recognizing antigen is that:
(Multiple Choice)
4.9/5  (30)
(30)
Individuals that overexpress the cytokine BAFF show increased susceptibility to autoimmune diseases such as Sjögren's syndrome, a disease that targets the exocrine glands that produce saliva, tears, and other bodily secretions. If one examined the circulating antibodies in these patients, one would expect to find:
(Multiple Choice)
4.9/5  (38)
(38)
If one swapped the regulatory elements of the TCR and TCR loci, so that rearrangement and expression of the TCR locus occurred first, in double-negative thymocytes, and TCR rearrangement and expression were delayed until the double-positive stage, would T cell development proceed normally to generate mature T cells? Why or why not?
(Essay)
4.8/5  (37)
(37)
Genetically inherited immunodeficiency diseases can result from defects in nearly any component of the immune response. The most severe forms of immunodeficiency occur when T cells are absent or non-functional. An individual with normal B cells, but an absence of T cells might have a defect in:
(Multiple Choice)
4.8/5  (37)
(37)
The pre-B-cell receptor provides an important signal that induces transition of pro-B cells to pre-B cells. An important characteristic of this receptor is that:
(Multiple Choice)
4.9/5  (34)
(34)
The repertoire of T-cell receptors, like that of antibodies, is formed by the random rearrangement of multiple gene segments that combine to generate the variable domain of each receptor subunit. The bias of T-cell receptors for binding to peptide:MHC complexes, rather than to all possible antigenic structures like antibodies, is simply the result of positive selection in the thymus.
(True/False)
4.8/5  (28)
(28)
Experimental mouse models have been developed to study the mechanisms leading to the breakdown of self-tolerance and the onset of autoimmunity. One strategy is to express a foreign antigen, such as a viral protein, in a single defined cell type in a peripheral organ. For instance, the lymphocytic choriomeningitis virus (LCMV) glycoprotein has been expressed in β-islet cells of the pancreas by making a line of mice that is transgenic for a construct linking the LCMV-glycoprotein gene to the insulin promoter. In these transgenic mice, the LCMV protein is expressed only in pancreatic -islet cells. Thymocytes with T-cell receptors specific for a peptide of LCMV-glycoprotein bound to MHC class I develop normally in the thymus, and do not undergo negative selection. The fate of these T cells once they emigrate from the thymus would likely be:
(Multiple Choice)
4.7/5  (39)
(39)
Two mutant lines of mice have been identified, each of which has a defect in T cell development. The subsets of thymocytes found in each mutant mouse line are shown in Figure
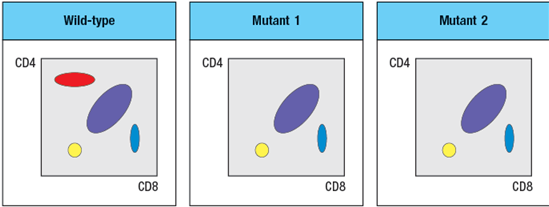 To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:
To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:
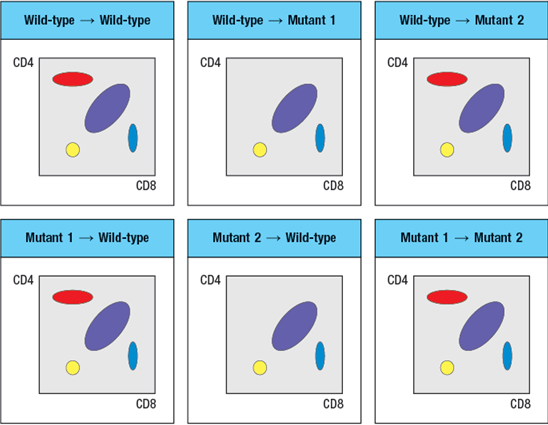 -A potential candidate molecule for the gene that is defective in Mutant-1 is:
-A potential candidate molecule for the gene that is defective in Mutant-1 is:
(Multiple Choice)
4.9/5  (24)
(24)
B and T lymphocytes develop from multipotent hematopoietic stem cells in the bone marrow. This process entails a continuum of development in which cells show progressive loss of multipotent potential, eventually becoming committed to a single lineage.
(True/False)
4.8/5  (28)
(28)
Self-reactive B cells can be eliminated from the repertoire at several stages of B cell maturation, including immature B cells that have already emigrated from the bone marrow into the circulation. This latter stage of tolerance induction is critical because:
(Multiple Choice)
4.8/5  (40)
(40)
Immature B cells expressing sIgM receptor emigrate from the bone marrow into the circulation. This is a passive process of cell diffusion, requiring no active signaling by the B cell.
(True/False)
4.9/5  (35)
(35)
Two mutant lines of mice have been identified, each of which has a defect in T cell development. The subsets of thymocytes found in each mutant mouse line are shown in Figure
 To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:
To narrow down the possible defects in each mutant line, a series of bone marrow chimeras are made in which bone marrow from one strain of mice (the ‘donor’ strain) is used to reconstitute a second strain (the ‘recipient’ strain), immediately following the irradiation of the recipient strain to eliminate its own hematopoietic cells. In this procedure, the resulting chimeras have hematopoietic cells that are 100% derived from the donor strain, and all other cells and tissues are derived from the recipient strain. The thymocyte profiles of the series of bone marrow chimeras is shown in Figure in each case the label at the top of each FACS plot refers to ‘donor bone marrow recipient’:
 -A potential candidate molecule for the gene that is defective in Mutant-2 is:
-A potential candidate molecule for the gene that is defective in Mutant-2 is:
(Multiple Choice)
4.8/5  (46)
(46)
Some specialized subsets of : T cells complete their development in the thymus and avoid negative selection, in spite of having T-cell receptors with high affinity for self-MHC complexes.
(True/False)
4.9/5  (32)
(32)
Synthesis question: To investigate how T-cell receptor signaling regulates T cell development in the thymus, a mutant mouse is generated with a deficiency in the T cell tyrosine kinase, LCK (Lck-/- mice). Analysis of thymocytes from these mice, stained with antibodies to CD4 and CD8, is shown in Figure.
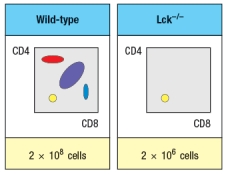 a) What is the explanation for the altered number and subsets of thymocytes in Lck-/- mice?
Due to the defect observed in the germline Lck-deficient mice, it is not possible to use these mice to examine any potential role for this T-cell receptor signaling protein at later stages of thymocyte development. To circumvent this problem, conditional Lck-deficient mice are generated by crossing mice with a homozygous 'floxed' allele of Lck (Lckfl/fl) to mice that express the cre recombinase in CD4+CD8+ double-positive thymocytes (i.e., CD4-cre). When cre is expressed at the double-positive stage, the Lck gene is deleted, and thymocytes become Lck-deficient at that time. The thymocyte profile of these Lckfl/fl x CD4-cre mice is shown in Figure.
a) What is the explanation for the altered number and subsets of thymocytes in Lck-/- mice?
Due to the defect observed in the germline Lck-deficient mice, it is not possible to use these mice to examine any potential role for this T-cell receptor signaling protein at later stages of thymocyte development. To circumvent this problem, conditional Lck-deficient mice are generated by crossing mice with a homozygous 'floxed' allele of Lck (Lckfl/fl) to mice that express the cre recombinase in CD4+CD8+ double-positive thymocytes (i.e., CD4-cre). When cre is expressed at the double-positive stage, the Lck gene is deleted, and thymocytes become Lck-deficient at that time. The thymocyte profile of these Lckfl/fl x CD4-cre mice is shown in Figure.
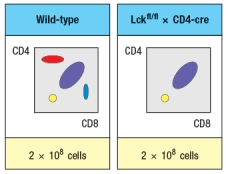 b) What is the explanation for the altered thymocyte profile seen in Lckfl/fl x CD4-cre mice?
To further assess the impact of altered T-cell receptor signaling on thymocyte development, another line of mice is generated that express a super-active version of the Lck kinase (called 'Lck-super') under control of the CD4 promoter. This super-active Lck is expressed starting at the double-positive stage in the thymus. Lck-super is activated by T-cell receptor signaling, just like wild-type Lck, but when activated has an approximately tenfold increased kinase activity. Surprisingly, mice expressing Lck-super do not develop increased numbers of mature T cells, but instead, show the following.
b) What is the explanation for the altered thymocyte profile seen in Lckfl/fl x CD4-cre mice?
To further assess the impact of altered T-cell receptor signaling on thymocyte development, another line of mice is generated that express a super-active version of the Lck kinase (called 'Lck-super') under control of the CD4 promoter. This super-active Lck is expressed starting at the double-positive stage in the thymus. Lck-super is activated by T-cell receptor signaling, just like wild-type Lck, but when activated has an approximately tenfold increased kinase activity. Surprisingly, mice expressing Lck-super do not develop increased numbers of mature T cells, but instead, show the following.
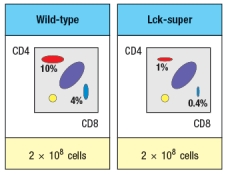 c) What is the most likely explanation for the altered profile of thymocytes seen in the Lck-super mice?
Struck by the findings in the Lck-super mice, an investigator performs one further experiment. This researcher clones the rearranged T-cell receptor and chain genes from two CD4 single-positive thymocytes: one that is isolated from a WT thymus, and the second, isolated from an Lck-super thymus. Each T-cell receptor : pair is used to generate a T-cell receptor transgenic line, so that nearly 100% of all double-positive thymocytes in each transgenic line express only the transgenic T-cell receptor. The two T cell receptor transgenics are named based on which mouse line the receptor chains were originally isolated from. The one from the wild-type line is known as TCR-tgwt, and the one originally isolated from the Lck-super line is known as TCR-tgsuper. In each case, thymocytes from the T-cell receptor transgenic lines are analyzed on a wild-type background, or after crossing to the Lck-super line. The results are shown in Figure.
c) What is the most likely explanation for the altered profile of thymocytes seen in the Lck-super mice?
Struck by the findings in the Lck-super mice, an investigator performs one further experiment. This researcher clones the rearranged T-cell receptor and chain genes from two CD4 single-positive thymocytes: one that is isolated from a WT thymus, and the second, isolated from an Lck-super thymus. Each T-cell receptor : pair is used to generate a T-cell receptor transgenic line, so that nearly 100% of all double-positive thymocytes in each transgenic line express only the transgenic T-cell receptor. The two T cell receptor transgenics are named based on which mouse line the receptor chains were originally isolated from. The one from the wild-type line is known as TCR-tgwt, and the one originally isolated from the Lck-super line is known as TCR-tgsuper. In each case, thymocytes from the T-cell receptor transgenic lines are analyzed on a wild-type background, or after crossing to the Lck-super line. The results are shown in Figure.
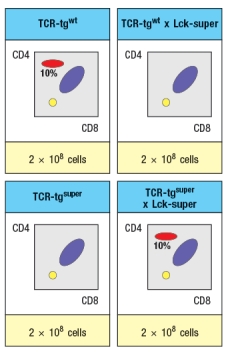 d) Explain the results observed in this experiment.
d) Explain the results observed in this experiment.
(Essay)
4.9/5  (41)
(41)
The final stages of T cell development occur in the thymic medulla, after the developing cells become CD4 or CD8 single-positive. One important change that occurs during this final maturation is:
(Multiple Choice)
4.9/5  (32)
(32)
B-1 B cells are considered a component of the innate rather than the adaptive immune response. The antibodies produced by B-1 B cells generally recognize capsular polysaccharide antigens found on many bacteria and viruses. These antibodies are considered part of the innate immune response because:
(Multiple Choice)
4.8/5  (47)
(47)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)