Deck 3: Stoichiometry: Mass, Formulas, and Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/141
Play
Full screen (f)
Deck 3: Stoichiometry: Mass, Formulas, and Reactions
1
A particular manufacturer sells phosphoric acid capsules as a homeopathic remedy with a concentration specified as 23X. (23X means that when averaged over many capsules, the average mass of phosphoric acid in one capsule is 10-23 times the mass of the capsule. The rest is a carbohydrate filler.) If each capsule has a mass of 35 mg, approximately how many capsules would you have to take to expect to ingest at least one molecule of H3PO4 (98.0 g/mol)?
A)1
B)5
C)50
D)500
E)5000
A)1
B)5
C)50
D)500
E)5000
500
2
In one analysis, 3.01 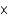 1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
A)5.00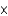 10-11 mol
10-11 mol
B)1.81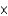 1037 mol
1037 mol
C)1.81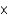 10-11 mol
10-11 mol
D)5.00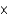 1037 mol
1037 mol
E)6.02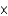 10-23 mol
10-23 mol
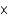 1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?A)5.00
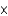 10-11 mol
10-11 molB)1.81
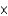 1037 mol
1037 molC)1.81
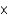 10-11 mol
10-11 molD)5.00
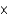 1037 mol
1037 molE)6.02
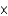 10-23 mol
10-23 mol5.00 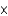 10-11 mol
10-11 mol
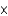 10-11 mol
10-11 mol 3
Which one of the following samples contains the largest number of molecules?
A)55 g of H2O
B)3 moles of H2O
C)3 moles of HF
D)62 g of H2O
E)62 g of HF
A)55 g of H2O
B)3 moles of H2O
C)3 moles of HF
D)62 g of H2O
E)62 g of HF
62 g of H2O
4
How many hydrogen atoms are there in a 346 g sample of pure ammonia (NH3)?
A)3.67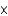 1025 atoms
1025 atoms
B)1.22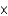 1025 atoms
1025 atoms
C)60.9 atoms
D)20.3 atoms
E)4.89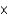 1025 atoms
1025 atoms
A)3.67
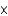 1025 atoms
1025 atomsB)1.22
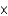 1025 atoms
1025 atomsC)60.9 atoms
D)20.3 atoms
E)4.89
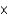 1025 atoms
1025 atoms
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
5
One mole is defined as ________
A)the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B)the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C)exactly 6.02 1022 particles.
1022 particles.
D)the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E)the number of particles equal to the number of atoms in exactly 1 kg of carbon in a vault at the National Bureau of Standards.
A)the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B)the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C)exactly 6.02
 1022 particles.
1022 particles.D)the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E)the number of particles equal to the number of atoms in exactly 1 kg of carbon in a vault at the National Bureau of Standards.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
6
A gallon of water has a mass of 3.79 kg. How many moles of water (18.02 g/mol) is this?
A)0.210 mol
B)210 mol
C)68.3 mol
D)68,300 mol
E)386 mol
A)0.210 mol
B)210 mol
C)68.3 mol
D)68,300 mol
E)386 mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
7
How many moles of ammonia are there in a 346 g sample of pure NH3 (17.03 g/mol)?
A)0.0496 mol
B)20.3 mol
C)24.7 mol
D)5,930 mol
E)3.46 mol
A)0.0496 mol
B)20.3 mol
C)24.7 mol
D)5,930 mol
E)3.46 mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement about the following chemical reaction is not correct? 2H2
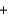
O2

2H2
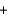
O2
A)For every oxygen molecule consumed, two water molecules are produced.
B)For every two hydrogen molecules consumed, two water molecules are produced.
C)Two hydrogen molecules react with one oxygen molecule.
D)Four hydrogen molecules react with oxygen to produce two water molecules.
E)Four hydrogen atoms combine with two oxygen atoms to produce two water molecules.
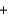
O2

2H2
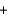
O2
A)For every oxygen molecule consumed, two water molecules are produced.
B)For every two hydrogen molecules consumed, two water molecules are produced.
C)Two hydrogen molecules react with one oxygen molecule.
D)Four hydrogen molecules react with oxygen to produce two water molecules.
E)Four hydrogen atoms combine with two oxygen atoms to produce two water molecules.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
9
In one analysis, the density of ozone in an air sample was found to be 5.00 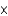 10-8 mol/L. What is the density in molecules per liter?
10-8 mol/L. What is the density in molecules per liter?
A)8.30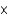 10-32
10-32
B)3.01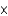 1016
1016
C)1.81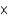 10-11
10-11
D)3.01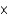 1011
1011
E)6.02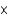 1023
1023
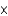 10-8 mol/L. What is the density in molecules per liter?
10-8 mol/L. What is the density in molecules per liter?A)8.30
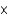 10-32
10-32B)3.01
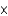 1016
1016C)1.81
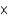 10-11
10-11D)3.01
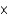 1011
1011E)6.02
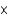 1023
1023
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
10
For a standard airbag, 131 g of sodium azide (65.00 g/mol) is required to generate enough gas to adequately fill the airbag. How many formula units of sodium azide are needed?
A)3.35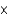 10-24 formula units
10-24 formula units
B)1.21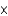 1024 formula units
1024 formula units
C)7.89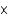 1025 formula units
1025 formula units
D)1.41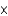 10-20 formula units
10-20 formula units
E)5.13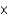 1027 formula units
1027 formula units
A)3.35
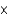 10-24 formula units
10-24 formula unitsB)1.21
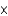 1024 formula units
1024 formula unitsC)7.89
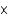 1025 formula units
1025 formula unitsD)1.41
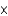 10-20 formula units
10-20 formula unitsE)5.13
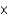 1027 formula units
1027 formula units
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
11
A sample of water (H2O) contains 1.81 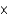 1024 molecules. How many total moles of atoms are there in this sample?
1024 molecules. How many total moles of atoms are there in this sample?
A)1.00
B)2.00
C)3.00
D)6.00
E)9.00
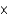 1024 molecules. How many total moles of atoms are there in this sample?
1024 molecules. How many total moles of atoms are there in this sample?A)1.00
B)2.00
C)3.00
D)6.00
E)9.00

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following contains the largest number of atoms?
A)1 mol water
B)1 mol phosphorus trichloride
C)1 mol dinitrogen pentoxide
D)1 mol carbon dioxide
E)All of these contain the same number of atoms.
A)1 mol water
B)1 mol phosphorus trichloride
C)1 mol dinitrogen pentoxide
D)1 mol carbon dioxide
E)All of these contain the same number of atoms.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
13
How many chlorine atoms are there in 16.0 g of carbon tetrachloride (CCl4)?
A)6.91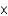 1025
1025
B)1.04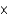 10-1
10-1
C)6.26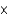 1022
1022
D)2.51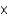 1023
1023
E)4.23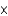 1017
1017
A)6.91
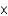 1025
1025B)1.04
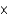 10-1
10-1C)6.26
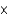 1022
1022D)2.51
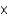 1023
1023E)4.23
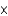 1017
1017
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement about the combustion of propane (C3H8) is not correct? C3H8
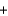
5O2

3CO2
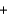
4H2O
A)For every propane molecule consumed, three molecules of carbon dioxide are produced.
B)For every two molecules of propane consumed, eight molecules of water are produced.
C)For every five molecules of oxygen consumed, four molecules of water are produced.
D)For every three molecules of carbon dioxide produced, five molecules of oxygen are consumed.
E)For every carbon dioxide molecule produced, four molecules of water are also produced.
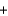
5O2

3CO2
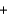
4H2O
A)For every propane molecule consumed, three molecules of carbon dioxide are produced.
B)For every two molecules of propane consumed, eight molecules of water are produced.
C)For every five molecules of oxygen consumed, four molecules of water are produced.
D)For every three molecules of carbon dioxide produced, five molecules of oxygen are consumed.
E)For every carbon dioxide molecule produced, four molecules of water are also produced.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
15
How many atoms of chlorine are there in 25.0 g of calcium chloride (CaCl2)?
A)1.36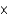 1023 atoms
1023 atoms
B)2.67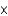 1024 atoms
1024 atoms
C)2.71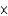 1023 atoms
1023 atoms
D)1.95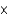 1023 atoms
1023 atoms
A)1.36
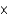 1023 atoms
1023 atomsB)2.67
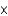 1024 atoms
1024 atomsC)2.71
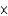 1023 atoms
1023 atomsD)1.95
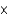 1023 atoms
1023 atoms
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement A-D regarding the terms mole and molar mass is not correct?
A)A mole is defined as the number of particles in exactly 12 g of carbon-12.
B)A mole of oxygen gas contains 6.022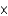 1023 molecules.
1023 molecules.
C)Two moles of oxygen atoms can be obtained by decomposing one mole of carbon dioxide.
D)To obtain the molar mass in grams (g/mol) from the atomic mass in atomic mass units(u), multiply by 6.022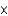 1023/mol and divide by 6.022
1023/mol and divide by 6.022 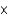 1023 u/g.
1023 u/g.
E)Statements A-D all are correct.
A)A mole is defined as the number of particles in exactly 12 g of carbon-12.
B)A mole of oxygen gas contains 6.022
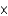 1023 molecules.
1023 molecules.C)Two moles of oxygen atoms can be obtained by decomposing one mole of carbon dioxide.
D)To obtain the molar mass in grams (g/mol) from the atomic mass in atomic mass units(u), multiply by 6.022
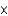 1023/mol and divide by 6.022
1023/mol and divide by 6.022 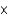 1023 u/g.
1023 u/g.E)Statements A-D all are correct.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
17
Which substance listed below contains the most oxygen atoms?
A)1 mol of Al2O3
B)1 mol of Fe2O3
C)2 mol of N2O4
D)2 mol of CO2
E)2 mol of HNO3
A)1 mol of Al2O3
B)1 mol of Fe2O3
C)2 mol of N2O4
D)2 mol of CO2
E)2 mol of HNO3

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
18
In one analysis, 1.34 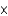 1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?
1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?
A)2.23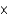 10-7 mol
10-7 mol
B)8.07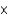 1040 mol
1040 mol
C)6.68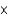 10-7 mol
10-7 mol
D)2.42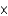 1041 mol
1041 mol
E)6.02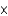 10-23 mol
10-23 mol
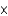 1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?
1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?A)2.23
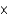 10-7 mol
10-7 molB)8.07
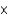 1040 mol
1040 molC)6.68
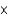 10-7 mol
10-7 molD)2.42
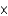 1041 mol
1041 molE)6.02
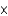 10-23 mol
10-23 mol
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
19
How many gold atoms are there in a 1.00 kg bar of gold?
A)3.06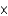 1024
1024
B)197 mol
C)3.06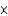 1021
1021
D)5.08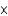 10-3 mol
10-3 mol
E)5.08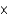 1018
1018
A)3.06
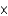 1024
1024B)197 mol
C)3.06
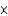 1021
1021D)5.08
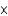 10-3 mol
10-3 molE)5.08
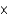 1018
1018
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
20
A tiny speck (8.3 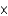 10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?
10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?
A)5.0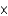 1017
1017
B)1.2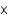 1020
1020
C)2.9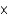 1022
1022
D)6.0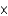 1023
1023
E)8.3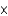 107
107
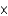 10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?
10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?A)5.0
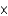 1017
1017B)1.2
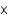 1020
1020C)2.9
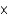 1022
1022D)6.0
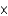 1023
1023E)8.3
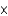 107
107
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
21
How many mL of hexadecane (C16H34) are needed to react with 0.050 mol O2, assuming complete combustion occurs? The density of C16H34 is 0.80 g/mL.
A)0.99 mL
B)0.37 mL
C)0.58 mL
D)0.46 mL
E)0.89 mL
A)0.99 mL
B)0.37 mL
C)0.58 mL
D)0.46 mL
E)0.89 mL

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement about the following chemical reaction is not correct? 2NH3 + 2O2 N2O + 3H2O
A)It requires 2 mol of dioxygen to produce 1 mol of N2O.
B)It requires 2 mol of ammonia to produce 3 mol of water.
C)Two moles of ammonia react with two moles of dioxygen.
D)Two moles of N2O would be produced when four moles of dioxygen are consumed.
E)Nine moles of water are produced when four moles of ammonia are consumed.
A)It requires 2 mol of dioxygen to produce 1 mol of N2O.
B)It requires 2 mol of ammonia to produce 3 mol of water.
C)Two moles of ammonia react with two moles of dioxygen.
D)Two moles of N2O would be produced when four moles of dioxygen are consumed.
E)Nine moles of water are produced when four moles of ammonia are consumed.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
23
Hydrogen peroxide decomposes to produce water and oxygen. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2H2O2 2H2O + O2
A)2 molecules 2 molecules +1 molecules
2 molecules +1 molecules
B)34.0 g 18.0 g
18.0 g 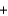 16.0 g
16.0 g
C)68.0 g 36.0 g
36.0 g 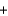 32.0 g
32.0 g
D)2x mol 2x mol
2x mol 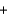 x mol
x mol
E)y(34.0 g) y(18.0 g)
y(18.0 g) 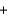 y(32 g)
y(32 g)
A)2 molecules
 2 molecules +1 molecules
2 molecules +1 moleculesB)34.0 g
 18.0 g
18.0 g 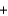 16.0 g
16.0 gC)68.0 g
 36.0 g
36.0 g 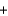 32.0 g
32.0 gD)2x mol
 2x mol
2x mol 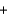 x mol
x molE)y(34.0 g)
 y(18.0 g)
y(18.0 g) 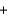 y(32 g)
y(32 g)
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
24
Fuming sulfuric acid is obtained by the addition of SO3 to concentrated H2SO4. The fumes result from the reaction of SO3 gas with water vapor. What is the product when one molecule of SO3 reacts with one molecule of water?
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)two molecules of sulfuric acid
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)two molecules of sulfuric acid

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
25
Air bags in cars inflate when an electrical spark activates sodium azide (NaN3) so that it decomposes to sodium metal, Na, and nitrogen gas, N2. In the balanced reaction equation, how many moles of nitrogen gas are formed for each mole of sodium azide?
A)1
B)1.5
C)2
D)3
E)2.5
A)1
B)1.5
C)2
D)3
E)2.5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
26
Sulfur dioxide from coal-fired power plants combines with water in the atmosphere to produce acid rain. What is the product when one molecule of SO2 reacts with one molecule of water?
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)one molecule of sulfurous acid
A)two molecules of sulfurous acid
B)one sulfate ion
C)two sulfite ions
D)one molecule of sulfuric acid
E)one molecule of sulfurous acid

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
27
Fe2O3(s) and powdered aluminum can react with great output of heat to form molten iron and Al2O3. When this reaction equation is balanced, what are the stoichiometric coefficients in the following order: Fe2O3, Al, Fe, Al2O3?
A)1, 1, 1, 1
B)2, 2, 2, 2
C)1, 2, 2, 1
D)2, 1, 1, 2
E)1, 1, 2, 2
A)1, 1, 1, 1
B)2, 2, 2, 2
C)1, 2, 2, 1
D)2, 1, 1, 2
E)1, 1, 2, 2

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
28
Nitrogen monoxide undergoes combustion to produce nitrogen dioxide. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2NO 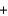 O2
O2  2NO2
2NO2
A)2x g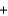 x g
x g  2x g
2x g
B)2 molecules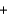 +1 molecule
+1 molecule  2 molecules
2 molecules
C)60.0 g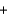 32.0 g
32.0 g  92.0 g
92.0 g
D)(2y)(30.0 g)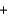 y (32.0 g)
y (32.0 g)  (2y)(46.0 g)
(2y)(46.0 g)
E)15.0 g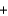 8.0 g
8.0 g  23.0 g
23.0 g
 O2
O2  2NO2
2NO2A)2x g
 x g
x g  2x g
2x gB)2 molecules
 +1 molecule
+1 molecule  2 molecules
2 moleculesC)60.0 g
 32.0 g
32.0 g  92.0 g
92.0 gD)(2y)(30.0 g)
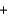 y (32.0 g)
y (32.0 g)  (2y)(46.0 g)
(2y)(46.0 g)E)15.0 g
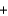 8.0 g
8.0 g  23.0 g
23.0 g
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
29
How many nitrogen atoms are there in 51.0 g of (NH4)2SO4 (ammonium sulfate)?
A)2.32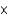 1023 atoms
1023 atoms
B)3.07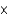 1025 atoms
1025 atoms
C)4.65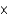 1023 atoms
1023 atoms
D)6.14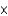 1025 atoms
1025 atoms
E)5.39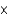 1023 atoms
1023 atoms
A)2.32
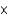 1023 atoms
1023 atomsB)3.07
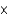 1025 atoms
1025 atomsC)4.65
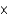 1023 atoms
1023 atomsD)6.14
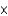 1025 atoms
1025 atomsE)5.39
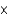 1023 atoms
1023 atoms
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
30
What is the molar mass of sulfuric acid (H2SO4)?
A)49.0 g/mol
B)24.5 g/mol
C)101 g/mol
D)98.1 g/mol
E)97.0 g/mol
A)49.0 g/mol
B)24.5 g/mol
C)101 g/mol
D)98.1 g/mol
E)97.0 g/mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following cartoons depict a balanced reaction?
A)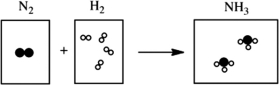
B)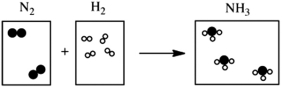
C)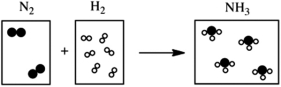
D)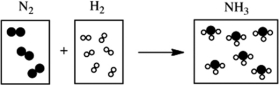
E)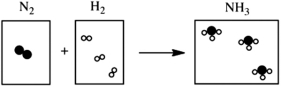
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
32
The acid-base reaction between phosphoric acid, H3PO4, and calcium hydroxide, Ca(OH)2, yields water and calcium phosphate. For each mole of calcium phosphate produced by this reaction, how many moles of water are produced?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
33
Which statement about the following chemical reaction is not correct? 3H2 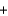
N2

2NH3
A)For every mole of nitrogen consumed, two moles of ammonia are produced.
B)For every two moles of nitrogen consumed, three moles of ammonia are produced.
C)For every three moles of hydrogen consumed, two moles of ammonia are produced.
D)For every two moles of ammonia produced, three moles of hydrogen are consumed.
E)Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.
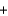
N2

2NH3
A)For every mole of nitrogen consumed, two moles of ammonia are produced.
B)For every two moles of nitrogen consumed, three moles of ammonia are produced.
C)For every three moles of hydrogen consumed, two moles of ammonia are produced.
D)For every two moles of ammonia produced, three moles of hydrogen are consumed.
E)Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
34
As a purchasing agent for a pharmaceutical company, how much chlorine, Cl2, do you need to order to react completely with 500 kg of platinum, Pt, to make cisplatin, PtCl2(NH3)2?
A)182 kg
B)500 kg
C)2,564 kg
D)364 kg
E)91 kg
A)182 kg
B)500 kg
C)2,564 kg
D)364 kg
E)91 kg

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement about a balanced chemical reaction equation is always correct?
A)The total number of moles of the products equals the total number of moles of the reactants.
B)The number of atoms of each kind is the same for the products as for the reactants.
C)The sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)The sum of the masses of gaseous reactants equals the sum of the masses of gaseous products.
E)The sum of the masses of solid products equals the sum of the masses of solid reactants.
A)The total number of moles of the products equals the total number of moles of the reactants.
B)The number of atoms of each kind is the same for the products as for the reactants.
C)The sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)The sum of the masses of gaseous reactants equals the sum of the masses of gaseous products.
E)The sum of the masses of solid products equals the sum of the masses of solid reactants.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
36
What is the molar mass of phosphorus pentachloride (PCl5)?
A)177.25 g/mol
B)190.30 g/mol
C)208.22 g/mol
D)172.75 g/mol
E)202.82 g/mol
A)177.25 g/mol
B)190.30 g/mol
C)208.22 g/mol
D)172.75 g/mol
E)202.82 g/mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
37
What is the formula mass of (NH4)3PO4 (ammonium phosphate)?
A)62.0 g/mol
B)113 g/mol
C)131 g/mol
D)149 g/mol
E)303 g/mol
A)62.0 g/mol
B)113 g/mol
C)131 g/mol
D)149 g/mol
E)303 g/mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following cartoons depict a balanced reaction?
A)
B)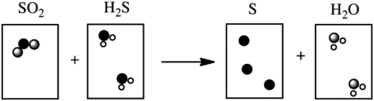
C)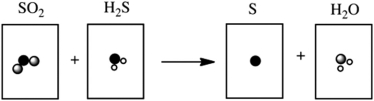
D)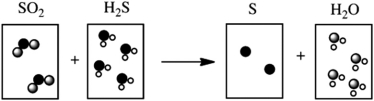
E)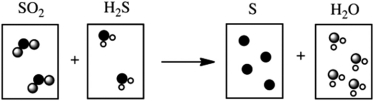
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
39
How many oxygen atoms are there in 27.0 g of sodium sulfate (Na2SO4)?
A)1.15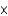 1023 atoms
1023 atoms
B)4.58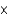 1023 atoms
1023 atoms
C)5.73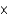 1023 atoms
1023 atoms
D)2.41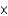 1024 atoms
1024 atoms
E)6.88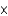 1023 atoms
1023 atoms
A)1.15
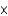 1023 atoms
1023 atomsB)4.58
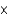 1023 atoms
1023 atomsC)5.73
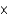 1023 atoms
1023 atomsD)2.41
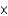 1024 atoms
1024 atomsE)6.88
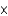 1023 atoms
1023 atoms
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the list below that has these chloride compounds arranged in order of increasing molar mass.
A)CCl4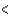 PCl3
PCl3
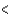 SCl2
SCl2  BCl3
BCl3
B)SCl2 BCl3
BCl3  PCl3
PCl3  CCl4
CCl4
C)BCl3 CCl4
CCl4  PCl3
PCl3  SCl2
SCl2
D)SCl2 BCl3
BCl3  CCl4
CCl4  PCl3
PCl3
E)BCl3 SCl2
SCl2  PCl3
PCl3  CCl4
CCl4
A)CCl4
 PCl3
PCl3  SCl2
SCl2  BCl3
BCl3B)SCl2
 BCl3
BCl3  PCl3
PCl3  CCl4
CCl4C)BCl3
 CCl4
CCl4  PCl3
PCl3  SCl2
SCl2D)SCl2
 BCl3
BCl3  CCl4
CCl4  PCl3
PCl3E)BCl3
 SCl2
SCl2  PCl3
PCl3  CCl4
CCl4
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
41
The combustion of ethanol (CH3CH2OH, 46.1 g/mol) results in the formation of water and carbon dioxide. How many grams of carbon dioxide are produced when 46.1 g of ethanol burns?
A)88.0 g
B)44.0 g
C)176.0 g
D)22.0 g
E)11.0 g
A)88.0 g
B)44.0 g
C)176.0 g
D)22.0 g
E)11.0 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
42
In a demonstration of the complete combustion of propane (C3H8) with oxygen (O2), several balloons were prepared with various proportions by volume of propane and oxygen. The loudest explosion occurred for the balloon with the correct stoichiometric proportions of the gases. Which balloon had the loudest explosion? Note that the number of molecules of a gas is proportional to the volume of the gas at a given pressure and temperature.
A)1 portion propane to 1 portion oxygen
B)1 portion propane to 3 portions oxygen
C)1 portion propane to 4 portions oxygen
D)1 portion propane to 5 portions oxygen
E)2 portions propane to 3 portions oxygen
A)1 portion propane to 1 portion oxygen
B)1 portion propane to 3 portions oxygen
C)1 portion propane to 4 portions oxygen
D)1 portion propane to 5 portions oxygen
E)2 portions propane to 3 portions oxygen

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
43
The average adult exhales about 1.0 kg of carbon dioxide each day. How much oxygen is needed in metabolizing glucose (C6H12O6, 180 g/mol) to make that much carbon dioxide?
A)180 g
B)1,800 g
C)360 g
D)730 g
E)1,500 g
A)180 g
B)1,800 g
C)360 g
D)730 g
E)1,500 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
44
Phosphorus trichloride reacts with water to form phosphorous acid, H3PO3, and hydrochloric acid in the following unbalanced reaction. For each mole of phosphorous acid produced by this reaction, how many moles of HCl are produced?__ PCl3 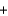 __H2O
__H2O  __H3PO3
__H3PO3 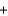 + __ HCl
+ __ HCl
A)1
B)2
C)3
D)4
E)5
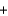 __H2O
__H2O  __H3PO3
__H3PO3 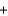 + __ HCl
+ __ HClA)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
45
Respiration is to photosynthesis as ________
A)a lake is to an ocean.
B)a plane is to a car.
C)Canada is to North America.
D)summer is to winter.
E)a cake is to a pie.
A)a lake is to an ocean.
B)a plane is to a car.
C)Canada is to North America.
D)summer is to winter.
E)a cake is to a pie.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
46
Which statement A-D regarding photosynthesis is not correct? In the process of photosynthesis ________
A)light energy is converted into carbon-based fuels.
B)oxygen is produced.
C)atmospheric carbon dioxide is consumed.
D)the sugar glucose is produced.
E)A-D are all correct.
A)light energy is converted into carbon-based fuels.
B)oxygen is produced.
C)atmospheric carbon dioxide is consumed.
D)the sugar glucose is produced.
E)A-D are all correct.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
47
Balance the following combustion reaction and report the sum of the stoichiometric coefficients.__ C4H8S2  __O2
__O2  __CO2
__CO2  __H2O
__H2O  __SO3
__SO3
A)16
B)17
C)18
D)19
E)20
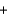 __O2
__O2  __CO2
__CO2 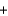 __H2O
__H2O 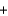 __SO3
__SO3A)16
B)17
C)18
D)19
E)20

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
48
What are the stoichiometric coefficients for oxygen and water, respectively, in the balanced chemical reaction equation representing the combustion of butane (C4H10)?
A)4, 5
B)9, 5
C)13, 10
D)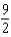 , 10
, 10
E)13, 5
A)4, 5
B)9, 5
C)13, 10
D)
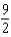 , 10
, 10E)13, 5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
49
Diesel fuel for automobiles and trucks is a mixture of hydrocarbons that can be modeled by C16H34. Write the balanced reaction equation for the combustion of C16H34, and report the sum of the stoichiometric coefficients.
A)4
B)120
C)76
D)83
E)117
A)4
B)120
C)76
D)83
E)117

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
50
The combustion of heptane (C7H16) forms carbon dioxide and water. What is the stoichiometric coefficient for water in the balanced equation when 1 mol of heptane undergoes combustion?
A)6
B)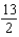
C)7
D)8
E)9
A)6
B)
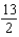
C)7
D)8
E)9

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
51
If the combustion of fossil fuels adds about 5 1012 kg of carbon to the atmosphere as CO2 each year, how much oxygen is required to produce this amount of carbon dioxide?
A)about 1.3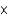 1012 kg
1012 kg
B)about 1.3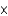 1013 kg
1013 kg
C)about 2.6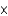 1012 kg
1012 kg
D)about 2.6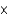 1013 kg
1013 kg
E)about 6.7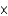 1012 kg
1012 kg
A)about 1.3
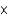 1012 kg
1012 kgB)about 1.3
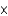 1013 kg
1013 kgC)about 2.6
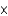 1012 kg
1012 kgD)about 2.6
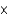 1013 kg
1013 kgE)about 6.7
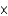 1012 kg
1012 kg
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
52
Ozone (O3) reacts with iodide (I-) and water to form iodine (I2), hydroxide (OH-), and oxygen (O2). Balance the following reaction equation and report the sum of the stoichiometric coefficients. O3 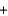 I-
I-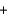 H2O
H2O  I2
I2 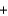 OH-
OH-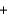 O2
O2
A)10
B)6
C)8
D)12
E)14
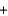 I-
I-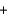 H2O
H2O  I2
I2 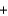 OH-
OH-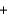 O2
O2A)10
B)6
C)8
D)12
E)14

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement A-D regarding the carbon cycle is not correct?
A)Petroleum, coal, and other carbon sediments are produced.
B)Respiration produces atmospheric carbon dioxide.
C)Water is a reactant in photosynthesis.
D)Forest fires are part of the carbon cycle.
E)A-D are all correct.
A)Petroleum, coal, and other carbon sediments are produced.
B)Respiration produces atmospheric carbon dioxide.
C)Water is a reactant in photosynthesis.
D)Forest fires are part of the carbon cycle.
E)A-D are all correct.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
54
One form of elemental sulfur is a ring of eight sulfur atoms. How many moles of molecular oxygen are consumed when one mole of this allotrope burns to make sulfur trioxide?
A)3
B)6
C)12
D)18
E)24
A)3
B)6
C)12
D)18
E)24

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
55
The combustion of fossil fuels is the main anthropogenic source of carbon dioxide in the atmosphere. During the combustion of gasoline in automobile engines, oxygen reacts with hydrocarbons to produce carbon dioxide and water. Assume gasoline is octane (C8H18). Write the balanced reaction equation, and report the sum of the stoichiometric coefficients written as integers.
A)55
B)29
C)31
D)30
E)61
A)55
B)29
C)31
D)30
E)61

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the set of stoichiometric coefficients that balances the reaction equation for the combustion of octane. C8H18 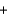 O2
O2  CO2
CO2 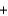 H2O
H2O
A)1, 25, 8, 9
B)1, 17, 8, 9
C)2, 34, 16, 18
D)2, 25, 16, 18
E)None of the above coefficients balance the reaction equation.
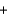 O2
O2  CO2
CO2 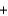 H2O
H2OA)1, 25, 8, 9
B)1, 17, 8, 9
C)2, 34, 16, 18
D)2, 25, 16, 18
E)None of the above coefficients balance the reaction equation.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
57
Ammonia undergoes combustion to produce nitrogen monoxide and water, both as gases. Write the balanced equation for this reaction and report the sum of the stoichiometric coefficients, written as integers.
A)17
B)13
C)23
D)21
E)19
A)17
B)13
C)23
D)21
E)19

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
58
Glucose (C6H12O6) is oxidized by molecular oxygen to carbon dioxide and water. How many O2 molecules are needed for each molecule of glucose that is oxidized?
A)1
B)2
C)6
D)12
E)18
A)1
B)2
C)6
D)12
E)18

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
59
A fuel cell works on the same principle as a battery but is continually fed with fuel. In one fuel cell, methanol (CH3OH) enters on one side of the unit and air enters on the other side. Both circulate past electrodes, and a chemical reaction occurs that produces electricity, plus carbon dioxide and water as byproducts. Which balanced chemical reaction equation best represents the reaction that occurs in this fuel cell?
A)CH3OH CO2
CO2  H2O
H2O
B)CH3OH CH3
CH3  OH
OH
C)CH3OH CH2
CH2  H2O
H2O
D)CH3OH O2
O2  CO2
CO2  H2O
H2O
E)2CH3OH 3O2
3O2  2CO2
2CO2  4H2O
4H2O
A)CH3OH
 CO2
CO2  H2O
H2OB)CH3OH
 CH3
CH3 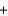 OH
OHC)CH3OH
 CH2
CH2 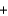 H2O
H2OD)CH3OH
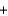 O2
O2  CO2
CO2 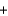 H2O
H2OE)2CH3OH
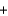 3O2
3O2  2CO2
2CO2 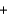 4H2O
4H2O
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
60
Burning coal that contains sulfur releases sulfur dioxide gas into the atmosphere, where it combines with water to form sulfurous and sulfuric acid, thereby producing acid rain. Assume sulfur in coal is in the form of pyrite (FeS2(s)), which reacts with molecular oxygen to produce Fe2O3(s) and SO2(g). Write the balanced equation for this reaction and report the sum of the stoichiometric coefficients, written as integers.
A)16
B)14
C)23
D)25
E)18
A)16
B)14
C)23
D)25
E)18

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
61
An iron ore, magnetite, contains only iron and oxygen. In refining 100.0 g of the ore, 72.35 g of iron are obtained. What is the empirical formula of the ore?
A)Fe2O3
B)FeO2
C)Fe2O5
D)Fe3O4
E)FeO
A)Fe2O3
B)FeO2
C)Fe2O5
D)Fe3O4
E)FeO

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
62
Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation. Na2CO3(s) 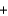 2C(s)
2C(s)  2Na(l)
2Na(l) 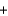 3CO(g)
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
A)0.50 kg
B)1.0 kg
C)1.5 kg
D)11 kg
E)21 kg
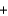 2C(s)
2C(s)  2Na(l)
2Na(l) 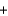 3CO(g)
3CO(g)If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
A)0.50 kg
B)1.0 kg
C)1.5 kg
D)11 kg
E)21 kg

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
63
Copper was the first metal to be produced from its ore because it is the easiest to smelt, that is, to refine by heating in the presence of carbon (hence the early occurrence of the Bronze Age). The ore was likely malachite (Cu2(OH)2CO3). What is the mass percent of copper in malachite?
A)28.7%
B)45.2%
C)57.5%
D)40.3%
E)74.6%
A)28.7%
B)45.2%
C)57.5%
D)40.3%
E)74.6%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
64
Baking ammonia or ammonium bicarbonate (NH4HCO3, 79.1 g/mol) is a leavening agent used in some older recipes. As it is heated, it breaks down into three gases: ammonia, water, and carbon dioxide. For each 20 g of baking ammonia heated (about 4 tsp), how many grams of carbon dioxide are produced?
A)15 g
B)7.5 g
C)11 g
D)22 g
E)20 g
A)15 g
B)7.5 g
C)11 g
D)22 g
E)20 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
65
One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis shows that the composition of chrysotile is 26.3% Mg, 20.2% Si, 1.45% H, and the remainder of the mass is oxygen. Determine the empirical formula of chrysotile.
A)MgSiHO3
B)Mg2Si2H2O5
C)Mg3Si2H4O6
D)Mg3Si2H4O9
E)Mg4Si3H5O9
A)MgSiHO3
B)Mg2Si2H2O5
C)Mg3Si2H4O6
D)Mg3Si2H4O9
E)Mg4Si3H5O9

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
66
Copper sulfate is a blue solid that is used to control algae growth. Solutions of copper sulfate that come in contact with the surface of galvanized (zinc-plated) steel pails undergo the following reaction that forms copper metal on the zinc surface. How many grams of zinc would react with 454 g (1 lb) of copper sulfate (160 g/mol)? CuSO4(aq) 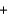 Zn(s)
Zn(s)  Cu(s)
Cu(s) 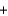 ZnSO4(aq)
ZnSO4(aq)
A)467 g
B)186 g
C)93 g
D)234 g
E)454 g
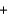 Zn(s)
Zn(s)  Cu(s)
Cu(s) 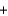 ZnSO4(aq)
ZnSO4(aq)A)467 g
B)186 g
C)93 g
D)234 g
E)454 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
67
A compound known to contain platinum, nitrogen, and hydrogen was analyzed and found to contain 74.1% Pt and 21.3% N; the remainder was hydrogen. What is the empirical formula for the compound?
A)Pt(NH3)4
B)Pt(NH3)3
C)Pt2(NH3)2
D)Pt(NH4)2
E)Pt(NH3)6
A)Pt(NH3)4
B)Pt(NH3)3
C)Pt2(NH3)2
D)Pt(NH4)2
E)Pt(NH3)6

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
68
Arizona was the site of a 400,000-acre wildfire in June 2002. How much carbon dioxide was produced by this fire? Assume that the density of carbon on the acreage was 10 kg/m2 and that 50% of the biomass burned. (10,000 m2 = 2.47 acre)
A)3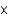 1010 kg
1010 kg
B)3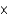 106 kg
106 kg
C)3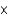 1030 kg
1030 kg
D)3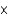 109 kg
109 kg
E)3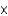 1013 kg
1013 kg
A)3
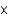 1010 kg
1010 kgB)3
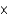 106 kg
106 kgC)3
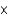 1030 kg
1030 kgD)3
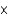 109 kg
109 kgE)3
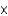 1013 kg
1013 kg
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
69
A range of organic molecules can undergo combustion. If pyridine (C5H5N) undergoes combustion through the following balanced chemical reaction, how much carbon dioxide can be produced when 3.2 g of pyridine reacts? 4C5H5N 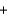 27O2
27O2
 H2O
H2O 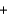 20CO2
20CO2 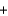 4NO
4NO
A)0.36 g
B)1.2 g
C)1.8 g
D)5.8 g
E)8.9 g
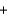 27O2
27O2  H2O
H2O 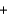 20CO2
20CO2 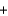 4NO
4NOA)0.36 g
B)1.2 g
C)1.8 g
D)5.8 g
E)8.9 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
70
Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O(l)  2H2(g)
2H2(g) 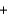 O2(g)
O2(g)
A)17.8 g
B)8.9 g
C)35.6 g
D)16.0 g
E)10.0 g
 2H2(g)
2H2(g) 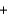 O2(g)
O2(g)A)17.8 g
B)8.9 g
C)35.6 g
D)16.0 g
E)10.0 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
71
Even though lead is toxic, lead compounds were used in ancient times as white pigments in cosmetics. What is the percentage of lead by mass in lead(IV) carbonate, Pb(CO3)2?
A)32.7%
B)20.7%
C)63.3%
D)77.5%
E)81.4%
A)32.7%
B)20.7%
C)63.3%
D)77.5%
E)81.4%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
72
What mass of phosphoric acid (H3PO4, 98.0 g/mol) is produced from the reaction of 10.0 g of P4O10 (284 g/mol) with excess water?
A)10.9 g
B)40.0 g
C)10.0 g
D)13.8 g
E)2.50 g
A)10.9 g
B)40.0 g
C)10.0 g
D)13.8 g
E)2.50 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
73
Baking soda (NaHCO3, 84.0 g/mol) requires acids from other ingredients to generate the carbon dioxide needed to make bread rise. The following equation describes this reaction, where HB is some unspecified acid. If 20.4 g of baking soda are used in a recipe and enough acid is present for a complete reaction, how many moles of carbon dioxide are generated? HB 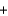 NaHCO3
NaHCO3  H2O
H2O 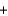 CO2
CO2 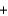 NaB
NaB
A)0.464 mol
B)0.334 mol
C)0.243 mol
D)0.204 mol
E)0.232 mol
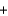 NaHCO3
NaHCO3  H2O
H2O 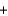 CO2
CO2 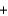 NaB
NaBA)0.464 mol
B)0.334 mol
C)0.243 mol
D)0.204 mol
E)0.232 mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
74
The average car emits 8.0 kg of carbon dioxide per gallon of combusted gas. What mass of gasoline (C8H18, 114.23 g/mol) was required to make that much carbon dioxide?
A)5.0 10-3 kg
B)1.3 kg
C)2.6 kg
D)21 kg
E)170 kg
A)5.0 10-3 kg
B)1.3 kg
C)2.6 kg
D)21 kg
E)170 kg

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
75
The most abundant metal in Earth's crust is aluminum, found mostly in the form of clays. There are no economical routes for extracting aluminum from clay. However, bauxite ore, impure hydrated aluminum oxide, is found in hot, humid regions, such as Australia, Guinea, and Brazil, and it can be purified and refined to make the metal. After the first purification step, hydrated aluminum oxide (Al2O3 . xH2O) is obtained. When 100.0 g of this solid were heated, and the water driven off, 65.36 g of Al2O3 remained. How many water molecules (x in the molecular formula) were there in the hydrate?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
76
Fool's Gold is the mineral pyrite (FeS2). What is the mass percent of sulfur in pyrite?
A)36.44%
B)46.59%
C)49.09%
D)53.41%
E)63.56%
A)36.44%
B)46.59%
C)49.09%
D)53.41%
E)63.56%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
77
Elemental analysis of the soot produced by a candle flame shows that it is 14.3% H and 85.7% C by mass. What is the empirical formula of this hydrocarbon?
A)CH
B)CH2
C)C2H
D)CH3
E)C2H3
A)CH
B)CH2
C)C2H
D)CH3
E)C2H3

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
78
Elemental analysis of the organic compound meta-xylene shows that it is 9.49% H and 90.51% C by mass. What is the empirical formula of this hydrocarbon?
A)CH
B)C2H3
C)C3H4
D)C4H5
E)C5H6
A)CH
B)C2H3
C)C3H4
D)C4H5
E)C5H6

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
79
A compound was analyzed and found to contain 25.24% S and 74.76% F. What is the empirical formula for the molecular compound?
A)SF
B)SF2
C)SF3
D)SF5
E)S2F4
A)SF
B)SF2
C)SF3
D)SF5
E)S2F4

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
80
Acetaminophen is a common medication used to treat pain and relieve fever. Mass analysis shows that the composition of acetaminophen is 63.56% C, 6.00% H, 9.27% N, and the remainder of the mass is oxygen. Determine the empirical formula of acetaminophen.
A)CHNO
B)C8H9NO2
C)C2H2NO
D)C3H4N2O3
E)C7H7N2O2
A)CHNO
B)C8H9NO2
C)C2H2NO
D)C3H4N2O3
E)C7H7N2O2

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck


