Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
In a demonstration of the complete combustion of propane (C3H8) with oxygen (O2), several balloons were prepared with various proportions by volume of propane and oxygen. The loudest explosion occurred for the balloon with the correct stoichiometric proportions of the gases. Which balloon had the loudest explosion? Note that the number of molecules of a gas is proportional to the volume of the gas at a given pressure and temperature.
Free
(Multiple Choice)
4.7/5  (27)
(27)
Correct Answer:
D
How many mL of hexadecane (C16H34) are needed to react with 0.050 mol O2, assuming complete combustion occurs? The density of C16H34 is 0.80 g/mL.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
What mass of phosphoric acid (H3PO4, 98.00 g/mol) is produced from the reaction of 10.00 g of P4O10 (283.89 g/mol) with 12.00 g water?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
D
One reaction that occurs during the deployment of an air bag is shown below, where sodium metal reacts with potassium nitrate. If 5.10 g of sodium and 305 g of potassium nitrate react in an airbag, how many grams of KNO3 remain because of the limited amount of sodium present? 10Na(s) 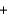 2KNO3(s)
2KNO3(s)  K2O(s)
K2O(s) 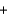 5Na2O(s)
5Na2O(s) 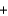 N2(g)
N2(g)
(Multiple Choice)
4.8/5  (36)
(36)
If the combustion of fossil fuels adds about 5 1012 kg of carbon to the atmosphere as CO2 each year, how much oxygen is required to produce this amount of carbon dioxide?
(Multiple Choice)
4.8/5  (36)
(36)
A range of organic molecules can undergo combustion. Pyridine (C5H5N) undergoes combustion in the unbalanced reaction shown below. Write the balanced equation, and find the percent yield for the reaction if 10.0 g of pyridine yields 18.1 g of carbon dioxide.
C5H5N 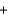 O2
O2
 H2O
H2O 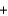 CO2
CO2 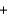 NO
NO
(Essay)
4.7/5  (38)
(38)
Arizona was the site of a 400,000-acre wildfire in June 2002. How much carbon dioxide was produced by this fire? Assume that the density of carbon on the acreage was 10 kg/m2 and that 50% of the biomass burned. (10,000 m2 = 2.47 acre)
(Multiple Choice)
4.8/5  (30)
(30)
Burning coal that contains sulfur releases sulfur dioxide gas into the atmosphere, where it combines with water to form sulfurous and sulfuric acid, thereby producing acid rain. Assume sulfur in coal is in the form of pyrite (FeS2(s)), which reacts with molecular oxygen to produce Fe2O3(s) and SO2(g). Write the balanced equation for this reaction and report the sum of the stoichiometric coefficients, written as integers.
(Multiple Choice)
4.8/5  (32)
(32)
In a self-contained breathing apparatus, potassium superoxide, KO2, reacts with carbon dioxide to form potassium carbonate and oxygen. Identify the limiting reactant and determine the amount of oxygen gas, in moles, that can be produced from 250 g of KO2 and 450 g of CO2. 4KO2(s) 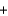 2CO2(g)
2CO2(g)  2K2CO3(s)
2K2CO3(s) 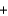 3O2(g)
3O2(g)
(Multiple Choice)
4.9/5  (31)
(31)
Methylheptenone (MHP) is used in producing lemon fragrances and flavors. It contains the elements carbon, hydrogen, and oxygen. The complete combustion of 192 mg of MHP produces 536 mg of carbon dioxide and 192 mg water. Mass spectrometry experiments showed that its molar mass is less than 200 g/mol. What is the molecular formula of MHP?
(Short Answer)
4.9/5  (27)
(27)
An iron ore, magnetite, contains only iron and oxygen. In refining 100.0 g of the ore, 72.35 g of iron are obtained. What is the empirical formula of the ore?
(Multiple Choice)
4.7/5  (32)
(32)
In one analysis, 3.01 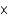 1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
1013 molecules of ozone were found in 1.00 mL of an air sample. How many moles of ozone is this?
(Multiple Choice)
4.9/5  (38)
(38)
Acetaminophen is a common medication used to treat pain and relieve fever. Mass analysis shows that the composition of acetaminophen is 63.56% C, 6.00% H, 9.27% N, and the remainder of the mass is oxygen. Determine the empirical formula of acetaminophen.
(Multiple Choice)
5.0/5  (35)
(35)
Azobenzene is a highly colored organic compound. Many diazo dyes used in coloring of clothing are based on an azobenzene core. The elemental analysis of azobenzene provides 79.10% carbon, 5.53% hydrogen, and the rest is nitrogen. It has a molar mass of 182.23 g/mol. What is the molecular formula of azobenzene?
(Multiple Choice)
4.8/5  (30)
(30)
For movie night, Annabelle popped two bags of popcorn. In Bag 1, 654 of a possible 1,213 kernels popped. In Bag 2, 343 of 690 kernels popped. Identify the bag with the highest popcorn yield and report this percent yield.
(Multiple Choice)
4.8/5  (40)
(40)
Balance the following combustion reaction and report the sum of the stoichiometric coefficients.__ C4H8S2 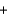 __O2
__O2  __CO2
__CO2 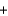 __H2O
__H2O 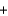 __SO3
__SO3
(Multiple Choice)
4.8/5  (33)
(33)
Like many metals, manganese reacts with halogens to give metal halides. How many grams of MnF3 can be produced using excess fluorine and 5.49 g of Mn? (molar masses: Mn = 54.9 g/mol,
F  19.0 g/mol)
2Mn(s)
19.0 g/mol)
2Mn(s)  3F2(g)
3F2(g)  2MnF3(s)
2MnF3(s)
(Short Answer)
4.8/5  (26)
(26)
Ammonia undergoes combustion to produce nitrogen monoxide and water, both as gases. Write the balanced equation for this reaction and report the sum of the stoichiometric coefficients, written as integers.
(Multiple Choice)
4.8/5  (38)
(38)
Which statements regarding combustion analysis to determine percent composition are not correct?
I. The mass of oxygen in the sample compound can be determined from the mass of carbon dioxide that is produced.
II. If a compound contains an element other than carbon and hydrogen, combustion analysis cannot be used to determine its empirical formula.
III. Combustion analysis data alone is not sufficient to determine the molecular formula with certainty.
IV. If some CO is produced rather than all CO2, then the empirical formula that is calculated will be too high in carbon.
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)