Deck 17: Equilibrium in the Aqueous Phase
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/156
Play
Full screen (f)
Deck 17: Equilibrium in the Aqueous Phase
1
When [H+] = 1.0 10-7 M in water at 25°C, then __________
A) pH = 1.
B) pH = 10-7.
C) [OH-] = 1.0 10-7 M.
D) [OH-] = 1.0 107 M.
E) [OH-] = 0 M.
A) pH = 1.
B) pH = 10-7.
C) [OH-] = 1.0 10-7 M.
D) [OH-] = 1.0 107 M.
E) [OH-] = 0 M.
[OH-] = 1.0 10-7 M.
2
Ammonia (NH3) acts as a weak base in aqueous solution. What is the acid that reacts with this base when ammonia is dissolved in water?
A) none, there are no acids in pure water
B) H2O
C) NH4+
D) trick question, because no acids are present, ammonia cannot act as a base
E) oxygen that always is dissolved in water
A) none, there are no acids in pure water
B) H2O
C) NH4+
D) trick question, because no acids are present, ammonia cannot act as a base
E) oxygen that always is dissolved in water
H2O
3
Which of these is a strong acid that ionizes to make a weak acid?
A) H2SO3
B) H2SO4
C) H3PO4
D) HNO3
E) HCl
A) H2SO3
B) H2SO4
C) H3PO4
D) HNO3
E) HCl
H2SO4
4
In the following reaction in aqueous solution, the acid reactant is __________, and its conjugate base product is __________. CH3NH2 + HSO4- 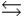 CH3NH3+ + SO42-
CH3NH3+ + SO42-
A) CH3NH2; CH3NH3+
B) CH3NH2; SO42-
C) HSO4-; CH3NH3+
D) HSO4-; SO42-
E) HSO4-; H3O+
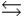 CH3NH3+ + SO42-
CH3NH3+ + SO42-A) CH3NH2; CH3NH3+
B) CH3NH2; SO42-
C) HSO4-; CH3NH3+
D) HSO4-; SO42-
E) HSO4-; H3O+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
5
In the Brønsted-Lowry definition of acids and bases, a base __________
A) is a proton donor.
B) is a proton acceptor.
C) forms stable hydrogen bonds.
D) breaks stable hydrogen bonds.
E) corrodes metals.
A) is a proton donor.
B) is a proton acceptor.
C) forms stable hydrogen bonds.
D) breaks stable hydrogen bonds.
E) corrodes metals.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
6
In the following reaction in aqueous solution, the acid reactant is __________ and its conjugate base product is __________. CH3COOH + NH3 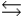 CH3COO- + NH4+
CH3COO- + NH4+
A) CH3COOH; CH3COO-
B) CH3COOH; NH4+
C) NH3; CH3COO-
D) NH3; NH4+
E) CH3COOH; H3O+
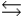 CH3COO- + NH4+
CH3COO- + NH4+A) CH3COOH; CH3COO-
B) CH3COOH; NH4+
C) NH3; CH3COO-
D) NH3; NH4+
E) CH3COOH; H3O+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
7
The degree of ionization of a weak acid __________
(I) varies with the concentration of the acid.
(II) depends on which weak acid it is.
(III) is 100%.
(IV) is greater than 50% but less than 100%.
A) I only
B) II only
C) III only
D) both I and II
E) IV only
(I) varies with the concentration of the acid.
(II) depends on which weak acid it is.
(III) is 100%.
(IV) is greater than 50% but less than 100%.
A) I only
B) II only
C) III only
D) both I and II
E) IV only

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
8
A solution with pH of 9.50 has a pOH of __________
A) 9.50.
B) 0.50.
C) 4.50.
D) 23.5.
E) 19.0.
A) 9.50.
B) 0.50.
C) 4.50.
D) 23.5.
E) 19.0.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
9
The base ionization constant Kb describes which of the following reactions for a weak base, B, in aqueous solution?
A) B + H+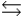 BH+
BH+
B) B + H3O+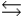 BH+ + H2O
BH+ + H2O
C) B + H2O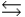 BH+ + OH-
BH+ + OH-
D) B + OH-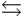 BH- + O2-
BH- + O2-
E) BH+ + OH- B + H2O
B + H2O
A) B + H+
 BH+
BH+B) B + H3O+
 BH+ + H2O
BH+ + H2OC) B + H2O
 BH+ + OH-
BH+ + OH-D) B + OH-
 BH- + O2-
BH- + O2-E) BH+ + OH-
 B + H2O
B + H2O
Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
10
The degree of ionization of a strong acid is __________
A) dependent on the concentration of the acid.
B) between 1 and 10%.
C) between 10 and 100%.
D) 100%.
E) dependent on which strong acid it is.
A) dependent on the concentration of the acid.
B) between 1 and 10%.
C) between 10 and 100%.
D) 100%.
E) dependent on which strong acid it is.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
11
Which one, A-D, is not related to the water autoionization constant, Kw? If all are related, respond E.
A) [H3O+] [OH-].
B) 1.0 10-14 at 25°C.
C) 2 H2O![<strong>Which one, A-D, is not related to the water autoionization constant, K<sub>w</sub>? If all are related, respond E.</strong> A) [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]. B) 1.0 <font face=symbol></font> 10<sup>-</sup><sup>14</sup> at 25°C. C) 2<sup> </sup>H<sub>2</sub>O H<sub>3</sub>O<sup>+</sup> + OH<sup>-</sup> D) pH = 7 at 25°C E) A-D are all related to K<sub>w</sub>.](https://storage.examlex.com/TB3833/11eaae02_088b_d27c_95d8_6d8c3d57e697_TB3833_11.jpg) H3O+ + OH-
H3O+ + OH-
D) pH = 7 at 25°C
E) A-D are all related to Kw.
A) [H3O+] [OH-].
B) 1.0 10-14 at 25°C.
C) 2 H2O
![<strong>Which one, A-D, is not related to the water autoionization constant, K<sub>w</sub>? If all are related, respond E.</strong> A) [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]. B) 1.0 <font face=symbol></font> 10<sup>-</sup><sup>14</sup> at 25°C. C) 2<sup> </sup>H<sub>2</sub>O H<sub>3</sub>O<sup>+</sup> + OH<sup>-</sup> D) pH = 7 at 25°C E) A-D are all related to K<sub>w</sub>.](https://storage.examlex.com/TB3833/11eaae02_088b_d27c_95d8_6d8c3d57e697_TB3833_11.jpg) H3O+ + OH-
H3O+ + OH-D) pH = 7 at 25°C
E) A-D are all related to Kw.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
12
The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid):
A) HA + OH- H2O + A-
H2O + A-
B) HA + H2O H3O+ + A-
H3O+ + A-
C) HA + H3O+ H2A+ + H2O
H2A+ + H2O
D) HA + H2A+ H2A+ + HA
H2A+ + HA
E) H3O+ + A- HA + H2O
HA + H2O
A) HA + OH-
 H2O + A-
H2O + A-B) HA + H2O
 H3O+ + A-
H3O+ + A-C) HA + H3O+
 H2A+ + H2O
H2A+ + H2OD) HA + H2A+
 H2A+ + HA
H2A+ + HAE) H3O+ + A-
 HA + H2O
HA + H2O
Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
13
Which sketch best represents the qualitative molecular view of an aqueous solution of nitric acid? (Water molecules are not shown explicitly.)
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
14
In the Brønsted-Lowry definition of acids and bases, an acid __________
A) is a proton donor.
B) is a proton acceptor.
C) forms stable hydrogen bonds.
D) breaks stable hydrogen bonds.
E) corrodes metals.
A) is a proton donor.
B) is a proton acceptor.
C) forms stable hydrogen bonds.
D) breaks stable hydrogen bonds.
E) corrodes metals.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement about nitrous acid and nitric acid is correct?
A) They are both weak acids.
B) They are both strong acids.
C) They both have one ionizable proton.
D) Nitrous acid has the formula HNO3.
E) Nitric acid has the formula HNO2.
A) They are both weak acids.
B) They are both strong acids.
C) They both have one ionizable proton.
D) Nitrous acid has the formula HNO3.
E) Nitric acid has the formula HNO2.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
16
A solution with a pOH of 6.92 has an [OH-] concentration of __________
A) 1.20 10-7 M.
B) 9.2 10-6 M.
C) 6.8 10-6 M.
D) 7.08 M.
E) 6.92 M.
A) 1.20 10-7 M.
B) 9.2 10-6 M.
C) 6.8 10-6 M.
D) 7.08 M.
E) 6.92 M.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
17
When [H+] = 4.0 10-9 M in water at 25°C, then __________
A) pH = 9.40.
B) pH = 7.00.
C) pH = -8.40.
D) pH = 8.40.
E) pH = -9.40
A) pH = 9.40.
B) pH = 7.00.
C) pH = -8.40.
D) pH = 8.40.
E) pH = -9.40

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
18
Which sketch best represents the qualitative molecular view of an aqueous solution of nitrous acid? (Water molecules are not shown explicitly.)
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
19
When pure water autoionizes, the following species are produced: __________
A) O2-, OH-, H3O+, and H2O+.
B) OH- and H3O+.
C) O2- and H4O2+.
D) H+ and OH-.
E) 2H+ and O2-.
A) O2-, OH-, H3O+, and H2O+.
B) OH- and H3O+.
C) O2- and H4O2+.
D) H+ and OH-.
E) 2H+ and O2-.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
20
Carbon dioxide in the atmosphere dissolves in rainwater to make rain __________
A) neutral.
B) acidic.
C) basic.
D) gray.
E) wet.
A) neutral.
B) acidic.
C) basic.
D) gray.
E) wet.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
21
A solution with a pOH of 4.3 has a [H+] of __________
A) 6.8 10-9 M.
B) 3.2 10-4 M.
C) 4.8 10-5 M.
D) 2.0 10-10 M.
E) 4.3 M.
A) 6.8 10-9 M.
B) 3.2 10-4 M.
C) 4.8 10-5 M.
D) 2.0 10-10 M.
E) 4.3 M.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
22
A substance that can act as both an acid and base is __________
A) amphibious.
B) amphiprotic.
C) bacidic.
D) androgynous.
E) acibasic
A) amphibious.
B) amphiprotic.
C) bacidic.
D) androgynous.
E) acibasic

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
23
Bert and Ernie were determining the pH of their goldfish's water. Bert's pH meter was in the fix-it shop, so Ernie used his pOH meter instead. Ernie insists that he can use the reading to calculate the pH by simple addition or subtraction that he learned in second grade, but Bert (showing off as usual) claims that the calculation involves finding an antilogarithm, dividing, and then finding a logarithm. This would make use of all the math he learned in third grade. Who is right?
A) Ernie
B) Bert
C) both
D) neither
A) Ernie
B) Bert
C) both
D) neither

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
24
In evaluating the pH of an aqueous weak acid solution, __________ usually can be ignored.
A) the concentration of the weak acid
B) the concentration of hydronium ion produced by the autoionization of water
C) the reaction of the weak acid with water
D) the concentration of the ionized hydronium ion
E) the concentration of the conjugate base
A) the concentration of the weak acid
B) the concentration of hydronium ion produced by the autoionization of water
C) the reaction of the weak acid with water
D) the concentration of the ionized hydronium ion
E) the concentration of the conjugate base

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
25
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O  H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka
H2PO4- + H2O HPO42- + H3O+ Ka
HPO42- + H3O+ Ka
 HPO42- + H2O
HPO42- + H2O 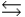 PO43- + H3O+ Ka
PO43- + H3O+ Ka
 What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
A)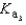
B)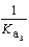
C)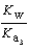
D)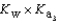
E)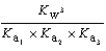
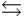 H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka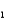
H2PO4- + H2O
 HPO42- + H3O+ Ka
HPO42- + H3O+ Ka HPO42- + H2O
HPO42- + H2O  PO43- + H3O+ Ka
PO43- + H3O+ Ka What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?A)
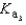
B)
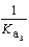
C)
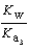
D)
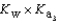
E)
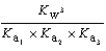

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
26
What is the actual concentration of the molecular form of HF in a 1.0 M HF solution given that Ka of HF is 6.8 10-4?
A) 2.6 10-2 M
B) 0.97 M
C) 1.59 M
D) 6.8 10-4 M
E) 1.0 M
A) 2.6 10-2 M
B) 0.97 M
C) 1.59 M
D) 6.8 10-4 M
E) 1.0 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
27
Pure water at any temperature has __________
A) a pH less than 7.
B) a pOH more than 7.
C) [H3O+] = [OH-].
D) pH = 7.
E) no hydronium ions in it.
A) a pH less than 7.
B) a pOH more than 7.
C) [H3O+] = [OH-].
D) pH = 7.
E) no hydronium ions in it.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
28
When values of Ka are small (e.g., 1 10-5) and concentrations of weak acids [HA] are relatively large (e.g., 0.10 M), the hydronium ion concentration of the solution can be calculated using which expression?
A) [H+] = Ka
B) [H+] = Ka[HA]
C)![<strong>When values of K<sub>a</sub> are small (e.g., 1 <font face=symbol></font> 10<sup>-</sup><sup>5</sup>) and concentrations of weak acids [HA] are relatively large (e.g., 0.10 M), the hydronium ion concentration of the solution can be calculated using which expression?</strong> A) [H<sup>+</sup>] = K<sub>a</sub> B) [H<sup>+</sup>] = K<sub>a</sub>[HA] C) D) [H<sup>+</sup>] = K<sub>a</sub>K<sub>b</sub>[HA] E) [H<sup>+</sup>] = K<sub>a</sub>[HA]/[A<sup>-</sup>]](https://storage.examlex.com/TB3833/11eaae02_088c_47ad_95d8_b93197e8ee25_TB3833_11.jpg)
D) [H+] = KaKb[HA]
E) [H+] = Ka[HA]/[A-]
A) [H+] = Ka
B) [H+] = Ka[HA]
C)
![<strong>When values of K<sub>a</sub> are small (e.g., 1 <font face=symbol></font> 10<sup>-</sup><sup>5</sup>) and concentrations of weak acids [HA] are relatively large (e.g., 0.10 M), the hydronium ion concentration of the solution can be calculated using which expression?</strong> A) [H<sup>+</sup>] = K<sub>a</sub> B) [H<sup>+</sup>] = K<sub>a</sub>[HA] C) D) [H<sup>+</sup>] = K<sub>a</sub>K<sub>b</sub>[HA] E) [H<sup>+</sup>] = K<sub>a</sub>[HA]/[A<sup>-</sup>]](https://storage.examlex.com/TB3833/11eaae02_088c_47ad_95d8_b93197e8ee25_TB3833_11.jpg)
D) [H+] = KaKb[HA]
E) [H+] = Ka[HA]/[A-]

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
29
What is the pOH of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
A) 4.44
B) 4.74
C) 0.70
D) 2.72
E) 3.38
A) 4.44
B) 4.74
C) 0.70
D) 2.72
E) 3.38

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
30
The degree of ionization __________
A) increases with increasing concentration of a weak acid.
B) decreases with increasing concentration of a weak acid.
C) does not change with changing concentration of a weak acid.
D) is not related to the concentration of a weak acid.
E) is independent of the composition of the weak acid.
A) increases with increasing concentration of a weak acid.
B) decreases with increasing concentration of a weak acid.
C) does not change with changing concentration of a weak acid.
D) is not related to the concentration of a weak acid.
E) is independent of the composition of the weak acid.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
31
What is the actual concentration of molecular NH3 in a 0.200 M solution of ammonia? The Kb value for ammonia is 1.80 10-5.
A) 0.200 M
B) 0.198 M
C) 1.80 10-5 M
D) 1.90 10-3 M
E) 3.6 10-6 M
A) 0.200 M
B) 0.198 M
C) 1.80 10-5 M
D) 1.90 10-3 M
E) 3.6 10-6 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
32
Sometimes liquid ammonia, NH3, is used as a solvent rather than water. Which expression defines the ammonia autoionization counterpart of Kw?
A) [H3O+][OH-]
B) [NH3][NH4+]
C) [NH2-][NH4+]
D) [H3O+][NH2-]
E) [NH4+][OH-]
A) [H3O+][OH-]
B) [NH3][NH4+]
C) [NH2-][NH4+]
D) [H3O+][NH2-]
E) [NH4+][OH-]

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
33
What is the hydronium ion concentration of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
A) 2.8 10-10
B) 5.5 10-10
C) 1.8 10-5
D) 5.2 10-12
E) 1.9 10-3
A) 2.8 10-10
B) 5.5 10-10
C) 1.8 10-5
D) 5.2 10-12
E) 1.9 10-3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
34
Which statement, A-D, is not correct? If all are correct, respond E. Pure water at 25°C has __________
A) Kw = 1.0 10-14.
B) pOH = 7.
C) [H3O+] = [OH-].
D) pH = 7.
E) A-D are all correct.
A) Kw = 1.0 10-14.
B) pOH = 7.
C) [H3O+] = [OH-].
D) pH = 7.
E) A-D are all correct.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
35
Which expression defines the autoionization constant for water, Kw?
A) [H3O+][OH-]
B) [H2O][H3O+]
C) [OH-][H2O]
D) [H4O2+][O2-]
E) [H2O][H2O]
A) [H3O+][OH-]
B) [H2O][H3O+]
C) [OH-][H2O]
D) [H4O2+][O2-]
E) [H2O][H2O]

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
36
What is the pH of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
A) 9.56
B) 9.26
C) 4.74
D) 11.28
E) 2.72
A) 9.56
B) 9.26
C) 4.74
D) 11.28
E) 2.72

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
37
What is the concentration of [OH-] in a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5.
A) 3.6 10-6 M
B) 1.8 10-5 M
C) 0.20 M
D) 1.9 10-3 M
E) 4.2 10-4 M
A) 3.6 10-6 M
B) 1.8 10-5 M
C) 0.20 M
D) 1.9 10-3 M
E) 4.2 10-4 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
38
What is the pH of a 0.010 M solution of acetic acid? Ka for acetic acid is 1.8 10-5
A) 2.74
B) 4.74
C) 2.00
D) 3.37
E) 6.74
A) 2.74
B) 4.74
C) 2.00
D) 3.37
E) 6.74

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
39
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 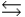 H2PO4- + H3O+
H2PO4- + H3O+ 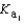 H2PO4- + H2O
H2PO4- + H2O 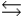 HPO42- + H3O+
HPO42- + H3O+ 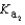 HPO42- + H2O
HPO42- + H2O 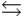 PO43- + H3O+
PO43- + H3O+ 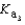 What is the Kb expression for the base, dihydrogen phosphate?
What is the Kb expression for the base, dihydrogen phosphate?
A)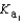
B)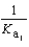
C)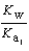
D)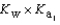
E)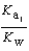
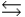 H2PO4- + H3O+
H2PO4- + H3O+ 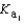 H2PO4- + H2O
H2PO4- + H2O  HPO42- + H3O+
HPO42- + H3O+ 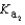 HPO42- + H2O
HPO42- + H2O  PO43- + H3O+
PO43- + H3O+ 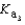 What is the Kb expression for the base, dihydrogen phosphate?
What is the Kb expression for the base, dihydrogen phosphate?A)
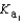
B)
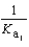
C)
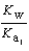
D)
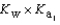
E)
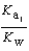

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
40
What is the hydronium ion concentration of a 0.010 M solution of acetic acid? Ka for acetic acid is 1.8 10-5
A) 1.8 10-3
B) 1.8 10-5
C) 1.0 10-2
D) 1.8 10-7
E) 4.2 10-4
A) 1.8 10-3
B) 1.8 10-5
C) 1.0 10-2
D) 1.8 10-7
E) 4.2 10-4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
41
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 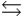 H2PO4- + H3O+
H2PO4- + H3O+ 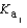 H2PO4- + H2O
H2PO4- + H2O 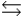 HPO42- + H3O+
HPO42- + H3O+ 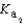 HPO42- + H2O
HPO42- + H2O 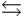 PO43- + H3O+
PO43- + H3O+ 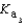 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
A) HPO42- + H2O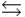 PO43- + H3O+
PO43- + H3O+
B) H3PO4 + H2O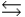 H2PO4- + H3O+
H2PO4- + H3O+
C) PO43- + H2O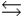 HPO42- + OH-
HPO42- + OH-
D) H2PO4- + H2O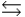 H3PO4 + OH-
H3PO4 + OH-
E) 2H2O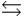 H3O+ + OH-
H3O+ + OH-
 H2PO4- + H3O+
H2PO4- + H3O+ 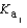 H2PO4- + H2O
H2PO4- + H2O  HPO42- + H3O+
HPO42- + H3O+ 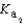 HPO42- + H2O
HPO42- + H2O 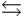 PO43- + H3O+
PO43- + H3O+ 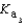 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?A) HPO42- + H2O
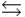 PO43- + H3O+
PO43- + H3O+B) H3PO4 + H2O
 H2PO4- + H3O+
H2PO4- + H3O+C) PO43- + H2O
 HPO42- + OH-
HPO42- + OH-D) H2PO4- + H2O
 H3PO4 + OH-
H3PO4 + OH-E) 2H2O
 H3O+ + OH-
H3O+ + OH-
Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
42
What is the solubility of barium sulfate in a solution containing 0.050 M sodium sulfate? The Ksp value for barium sulfate is 1.1 10-10.
A) 7.4 10-6 M
B) 5.5 10-11 M
C) 1.0 10-5 M
D) 2.2 10-9 M
E) 1.1 10-10 M
A) 7.4 10-6 M
B) 5.5 10-11 M
C) 1.0 10-5 M
D) 2.2 10-9 M
E) 1.1 10-10 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
43
Suppression of the solubility of one ion by the addition of the counter-ion in its insoluble salt is known as the __________
A) ionic suppression effect.
B) counter ion effect.
C) common ion effect.
D) excession effect.
E) supersaturation effect.
A) ionic suppression effect.
B) counter ion effect.
C) common ion effect.
D) excession effect.
E) supersaturation effect.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following salts forms aqueous solutions with pH = 7?
A) Na2S
B) NaBr
C) NaClO2
D) NaNO2
E) Na2CO3
A) Na2S
B) NaBr
C) NaClO2
D) NaNO2
E) Na2CO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
45
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate). The Ksp of calcium carbonate is 4.5 10-9. What is the concentration of a saturated calcium carbonate solution?
A) 0.00104 M
B) 4.5 10-9 M
C) 6.7 10-5 M
D) 2.25 10-9 M
E) 4.5 10-5 M
A) 0.00104 M
B) 4.5 10-9 M
C) 6.7 10-5 M
D) 2.25 10-9 M
E) 4.5 10-5 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
46
Aqueous solutions of __________ are basic.
A) NaF
B) NaCl
C) NaBr
D) NaI
E) KI
A) NaF
B) NaCl
C) NaBr
D) NaI
E) KI

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
47
The solubility product for an insoluble salt with the formula M3X is written as __________, where s is the molar solubility.
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 27s4
D) Ksp = 3s3
E) Ksp = 3s4
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 27s4
D) Ksp = 3s3
E) Ksp = 3s4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following salts forms aqueous solutions with pH = 7?
A) Na2S
B) NaCl
C) NaClO2
D) NaNO2
E) Na2CO3
A) Na2S
B) NaCl
C) NaClO2
D) NaNO2
E) Na2CO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
49
The solubility product for an insoluble salt with the formula M2X is written as __________, where s is the molar solubility.
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 4s2
D) Ksp = 2s3
E) Ksp = 2s2
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 4s2
D) Ksp = 2s3
E) Ksp = 2s2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
50
The solubility product for an insoluble salt with the formula MX2 is written as __________, where s is the molar solubility.
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 4s2
D) Ksp = 2s3
E) Ksp = 2s2
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 4s2
D) Ksp = 2s3
E) Ksp = 2s2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
51
Which one of the following salts does not produce a basic solution when dissolved in water?
A) Na2S
B) NaBr
C) NaClO2
D) NaNO2
E) Na2CO3
A) Na2S
B) NaBr
C) NaClO2
D) NaNO2
E) Na2CO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
52
The solubility product for an insoluble salt with the formula MX3 is written as __________, where s is the molar solubility.
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 27s4
D) Ksp = 3s3
E) Ksp = 3s4
A) Ksp = s2
B) Ksp = 4s3
C) Ksp = 27s4
D) Ksp = 3s3
E) Ksp = 3s4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
53
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 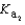 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
A) nearly 14
B) slightly above 7.00
C) 7.00
D) slightly below 7.00
E) nearly 1.00
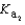 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.A) nearly 14
B) slightly above 7.00
C) 7.00
D) slightly below 7.00
E) nearly 1.00

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
54
The pH of an aqueous sodium fluoride (NaF) solution is __________ because __________
A) 7; sodium fluoride is a simple salt.
B) above 7; fluoride is a weak base.
C) below 7; fluoride reacts with water to make hydrofluoric acid.
D) about 7; fluoride is a weak base, but produces hydrofluoric acid, and these two neutralize one another.
E) 0; sodium fluoride is a salt not an acid or a base.
A) 7; sodium fluoride is a simple salt.
B) above 7; fluoride is a weak base.
C) below 7; fluoride reacts with water to make hydrofluoric acid.
D) about 7; fluoride is a weak base, but produces hydrofluoric acid, and these two neutralize one another.
E) 0; sodium fluoride is a salt not an acid or a base.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
55
The stronger the acid, __________
A) the stronger its conjugate base.
B) the weaker its conjugate base.
C) the more concentrated the acid.
D) the less concentrated the conjugate base.
E) the more concentrated the conjugate base.
A) the stronger its conjugate base.
B) the weaker its conjugate base.
C) the more concentrated the acid.
D) the less concentrated the conjugate base.
E) the more concentrated the conjugate base.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
56
If 500 mL of a solution containing 1.00 g of barium nitrate (199.33 g/mol) is combined with 500 mL of a solution containing 1.00 g of sodium sulfate (142.03 g/mol), will there be a precipitate? Barium sulfate has a Ksp value of 9.1 10-11.
A) Barium sulfate will precipitate.
B) There will be no precipitate.
C) It is impossible to tell.
D) Sodium nitrate will precipitate.
E) Barium nitrate will precipitate.
A) Barium sulfate will precipitate.
B) There will be no precipitate.
C) It is impossible to tell.
D) Sodium nitrate will precipitate.
E) Barium nitrate will precipitate.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
57
What is the pH of a 0.15 M solution of ammonium bromide? The Kb value for ammonia is 1.8 10-5.
A) 11.22
B) 7.00
C) 2.78
D) 5.04
E) 10.08
A) 11.22
B) 7.00
C) 2.78
D) 5.04
E) 10.08

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
58
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 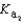 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
A) 7.00
B) 6.85
C) 7.15
D) 3.55
E) 12.22
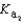 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.A) 7.00
B) 6.85
C) 7.15
D) 3.55
E) 12.22

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
59
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate). The Ksp of calcium carbonate is 4.5 10-9. What is the minimum volume of dripping water saturated with calcium carbonate that would be required to form a small stalactite that had a mass of 1.000 kg?
A) 1.4 106 L
B) 1.5 105 L
C) 4.5 109 L
D) 4.5 104 L
E) 1.5 104 L
A) 1.4 106 L
B) 1.5 105 L
C) 4.5 109 L
D) 4.5 104 L
E) 1.5 104 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
60
When sodium chloride is added to a saturated solution of lead(II) chloride, some of the lead(II) chloride precipitates. This phenomenon is called __________
A) the common ion effect.
B) selective precipitation.
C) supersaturation.
D) a solubility anomaly.
E) deionization.
A) the common ion effect.
B) selective precipitation.
C) supersaturation.
D) a solubility anomaly.
E) deionization.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds would not have a pH dependent solubility?
A) Mg(OH)2
B) PbS
C) AgI
D) Na2O
E) pbS
A) Mg(OH)2
B) PbS
C) AgI
D) Na2O
E) pbS

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
62
Acid-base indicators need to have very intense colors so that a very low concentration is visible. Could there be a problem in using too much indicator?
A) No, the colors would just be darker.
B) No, indicators are inert.
C) Yes, more indicator requires more extreme pH values to change color.
D) Yes, the indicator could affect the acid-base chemistry being measured.
E) No, the colors would just be sharper.
A) No, the colors would just be darker.
B) No, indicators are inert.
C) Yes, more indicator requires more extreme pH values to change color.
D) Yes, the indicator could affect the acid-base chemistry being measured.
E) No, the colors would just be sharper.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
63
A phosphate buffer solution (25.00 mL sample) used for a growth medium was titrated with 0.1000 M hydrochloric acid. The components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. The first endpoint occurred at a volume of 10.32 mL; the second occurred after an additional 18.62 mL was added (or total volume = 28.94 mL). What was the approximate ratio of HPO42- to H2PO4- in the buffer?
A) 1:1
B) 4:5
C) 5:3
D) 5:4
E) 3:5
A) 1:1
B) 4:5
C) 5:3
D) 5:4
E) 3:5

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
64
As the pH decreases, the solubility of __________ would increase.
A) lead(II) chloride
B) silver(I) iodate
C) calcium carbonate
D) mercury(I) bromide
E) silver(I) chloride
A) lead(II) chloride
B) silver(I) iodate
C) calcium carbonate
D) mercury(I) bromide
E) silver(I) chloride

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is not a buffer system? A solution containing roughly equal concentrations of __________
A) fluoride ion and hydrofluoric acid.
B) bromide ion and hydrobromic acid.
C) phosphate ion and hydrogen phosphate ion.
D) carbonate ion and hydrogen carbonate ion.
E) phosphoric acid and dihydrogen phosphate ion.
A) fluoride ion and hydrofluoric acid.
B) bromide ion and hydrobromic acid.
C) phosphate ion and hydrogen phosphate ion.
D) carbonate ion and hydrogen carbonate ion.
E) phosphoric acid and dihydrogen phosphate ion.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
66
A solution that contains a weak acid and its conjugate base in roughly equal concentrations is __________
A) neither acidic or basic.
B) a half-acid solution.
C) a buffer.
D) a heterogeneous mixture.
E) neutral.
A) neither acidic or basic.
B) a half-acid solution.
C) a buffer.
D) a heterogeneous mixture.
E) neutral.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
67
The following titration curve is most likely to be associated with 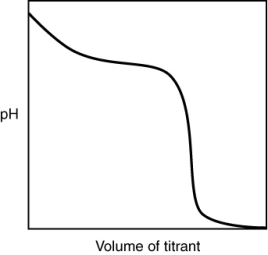
A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.

A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
68
The most suitable acid-base indicator for a titration of acetic acid with NaOH has __________
A) a pKin = pKa of the acid.
B) a pKin = pKb of the base.
C) pKin = pH of a sodium acetate solution.
D) pKin = pH of an acetic acid solution.
E) pKin = 7.0
A) a pKin = pKa of the acid.
B) a pKin = pKb of the base.
C) pKin = pH of a sodium acetate solution.
D) pKin = pH of an acetic acid solution.
E) pKin = 7.0

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
69
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution. Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution. The resulting titration curves are illustrated here. Given the following possibilities, what is the sample? 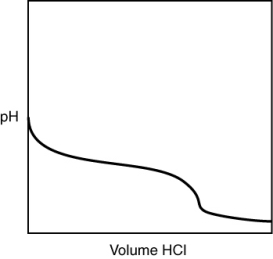
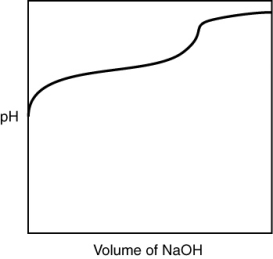
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.


A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
70
Halfway to the equivalence point in a titration curve of a weak acid with a strong base, __________
A) nothing is happening yet.
B) the pH = pKa of the weak acid.
C) pH = 3.5 exactly.
D) pH = pKa of the indicator.
E) the pH has not yet changed.
A) nothing is happening yet.
B) the pH = pKa of the weak acid.
C) pH = 3.5 exactly.
D) pH = pKa of the indicator.
E) the pH has not yet changed.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
71
What are the characteristics of a pH indicator? A pH indicator __________
(I) is a weak acid.
(II) has different characteristic colors in protonated and unprotonated forms.
(III) changes color when the pH is near its pKa.
A) I only
B) II only
C) both II and III
D) I, II, and III
E) I and III
(I) is a weak acid.
(II) has different characteristic colors in protonated and unprotonated forms.
(III) changes color when the pH is near its pKa.
A) I only
B) II only
C) both II and III
D) I, II, and III
E) I and III

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
72
When an acetic acid solution is titrated with sodium hydroxide, the slope of the titration curve (pH vs volume of NaOH added) increases when sodium hydroxide is first added. This change shows that __________.
A) nothing is happening during this part of the titration.
B) the reaction is very slow during this part of the titration.
C) a more concentrated solution of NaOH needs to be present to initiate the reaction.
D) acetic acid is being converted to sodium acetate.
E) the pH is not affected until all the acetic acid is consumed.
A) nothing is happening during this part of the titration.
B) the reaction is very slow during this part of the titration.
C) a more concentrated solution of NaOH needs to be present to initiate the reaction.
D) acetic acid is being converted to sodium acetate.
E) the pH is not affected until all the acetic acid is consumed.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
73
The following titration curve is most likely to be associated with __________ 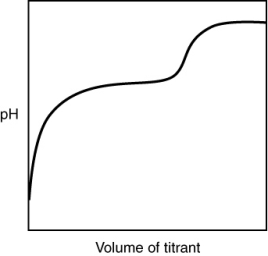
A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.

A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
74
A solution of sulfuric acid (H2SO4, 25.00 mL) was titrated to completion with 34.55 mL of 0.1020 M sodium hydroxide. What was the concentration of the sulfuric acid?
A) 0.07048 M
B) 0.1410 M
C) 0.2819 M
D) 0.0353 M
E) 0.0533 M
A) 0.07048 M
B) 0.1410 M
C) 0.2819 M
D) 0.0353 M
E) 0.0533 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
75
In a titration of monoprotic acids and bases, there is a large change in pH __________
A) at the point where pH = pKa of the acid.
B) when the volume of acid is exactly equal to the volume of base.
C) when the concentration of acid is exactly equal to the concentration of base.
D) when the number of moles of acid is exactly equal to the number of moles of base.
E) at the point where pH = pKb of the base.
A) at the point where pH = pKa of the acid.
B) when the volume of acid is exactly equal to the volume of base.
C) when the concentration of acid is exactly equal to the concentration of base.
D) when the number of moles of acid is exactly equal to the number of moles of base.
E) at the point where pH = pKb of the base.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
76
What is indicated by the shape of the titration curve? 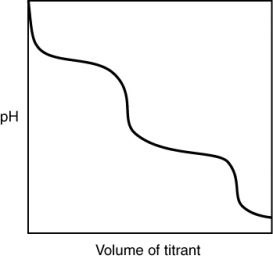
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.

A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
77
A solution of hydrochloric acid (HCl, 25.00 mL) was titrated to completion with 34.55 mL of 0.1020 M sodium hydroxide. What was the concentration of the hydrochloric acid?
A) 0.07048 M
B) 0.1410 M
C) 0.2819 M
D) 0.0353 M
E) 0.0533 M
A) 0.07048 M
B) 0.1410 M
C) 0.2819 M
D) 0.0353 M
E) 0.0533 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
78
A phosphate buffer solution (25.00 mL sample) used for a growth medium was titrated with 0.1000 M hydrochloric acid. The components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. The first endpoint occurred at a volume of 10.32 mL, and the second occurred after an additional 18.62 mL was added, for a total volume of 28.94 mL. What was the total concentration of phosphate (in any form) in the buffer?
A) 0.03992 M
B) 0.1198 M
C) 0.04243 M
D) 0.07448 M
E) 0.08382 M
A) 0.03992 M
B) 0.1198 M
C) 0.04243 M
D) 0.07448 M
E) 0.08382 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
79
Bromocresol green is yellow in its acidic form and blue in its basic form. When is it green?
A) at the equivalence point in a titration
B) in its neutral form
C) when the solution pH equals its pKa
D) when the solution pH equals 7
E) at the midpoint in a titration
A) at the equivalence point in a titration
B) in its neutral form
C) when the solution pH equals its pKa
D) when the solution pH equals 7
E) at the midpoint in a titration

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
80
Acid-base indicators change color __________
A) exactly when pH = pKa of the indicator.
B) generally over a range of 1 or 2 pH units.
C) at pH = 7.
D) always between a pH of 6 and 8.
E) at the midpoint of a titration.
A) exactly when pH = pKa of the indicator.
B) generally over a range of 1 or 2 pH units.
C) at pH = 7.
D) always between a pH of 6 and 8.
E) at the midpoint of a titration.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck


