Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 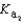 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
C
Purveyors of salts from the Dead Sea advertise that it is healthy to bathe in a saturated solution of magnesium chloride (MgCl2, 95.21 g/mol, Ksp = 740). How much magnesium chloride would you have to purchase to make up 10.0 L of bath water saturated with magnesium chloride?
Free
(Multiple Choice)
4.8/5  (54)
(54)
Correct Answer:
D
Research with biochemical systems commonly requires buffers because __________
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
If 500 mL of a solution containing 1.00 g of barium nitrate (199.33 g/mol) is combined with 500 mL of a solution containing 1.00 g of sodium sulfate (142.03 g/mol), will there be a precipitate? Barium sulfate has a Ksp value of 9.1 10-11.
(Multiple Choice)
4.7/5  (32)
(32)
Which one of the following salts forms aqueous solutions with pH = 7?
(Multiple Choice)
4.9/5  (36)
(36)
To simulate the pH of blood, which is 7.4, an undergraduate researcher in a biology lab produced a buffer solution by dissolving sodium dihydrogen phosphate (Ka = 6.2 10-8) and sodium hydrogen phosphate (Ka = 3.6 10-13) together in an aqueous solution. What mole ratio of Na2HPO4/NaH2PO4 did she need to use?
(Short Answer)
5.0/5  (39)
(39)
Which combination of solutions is the best choice for making a buffer solution?
(Multiple Choice)
4.8/5  (30)
(30)
Which one, A-D, is not related to the water autoionization constant, Kw? If all are related, respond E.
(Multiple Choice)
4.9/5  (31)
(31)
A solution of hydrochloric acid (HCl, 25.00 mL) was titrated to completion with 34.55 mL of 0.1020 M sodium hydroxide. What was the concentration of the hydrochloric acid?
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following groups, A-D, consist of salts that all form basic solutions in water? (Ac = acetate) If none or all satisfy this criterion, respond E.
(Multiple Choice)
4.7/5  (32)
(32)
A 25.0 mL solution of quinine was titrated with 1.00 M hydrochloric acid, HCl. It was found that the solution contained 0.125 moles of quinine. What was the pH of the solution after 50.00 mL of the HCl solution were added? Quinine is monobasic with pKb = 5.10.
(Multiple Choice)
4.7/5  (29)
(29)
Which of these is a strong acid that ionizes to make a weak acid?
(Multiple Choice)
4.8/5  (37)
(37)
Ammonia (NH3) acts as a weak base in aqueous solution. What is the acid that reacts with this base when ammonia is dissolved in water?
(Multiple Choice)
4.9/5  (45)
(45)
What is the actual concentration of the molecular form of HF in a 1.0 M HF solution given that Ka of HF is 6.8 10-4?
(Multiple Choice)
4.8/5  (37)
(37)
What is the pH of a 0.500 M solution of trimethylamine (pKb = 4.13)?
(Multiple Choice)
4.8/5  (35)
(35)
A substance that can act as both an acid and base is __________
(Multiple Choice)
5.0/5  (40)
(40)
Which one of the following is not a conjugate acid-base pair?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)