Deck 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy
1
"Ssys" can be directly related to the heat q. Which of A-D is not true regarding this relationship? If all are true, select E.
A) "Ssys" can always be determined from the heat transferred during the actual process.
B) For a reversible spontaneous endothermic process, both q and "Ssys" will be positive.
C) The more heat that is transferred, the larger the magnitude of the entropy change.
D) The higher the temperature at which heat is transferred, the lower the entropy change.
E) All of the above are true.
A) "Ssys" can always be determined from the heat transferred during the actual process.
B) For a reversible spontaneous endothermic process, both q and "Ssys" will be positive.
C) The more heat that is transferred, the larger the magnitude of the entropy change.
D) The higher the temperature at which heat is transferred, the lower the entropy change.
E) All of the above are true.
"Ssys" can always be determined from the heat transferred during the actual process.
2
Which of the following processes are spontaneous?
(I)Iron in the open air rusts.
(II)Liquid water in a freezer turns to ice.
(III)A spark ignites a mixture of propane and air.
A) I only
B) I and II only
C) I and III only
D) II and III only
E) I, II, and III are all spontaneous.
(I)Iron in the open air rusts.
(II)Liquid water in a freezer turns to ice.
(III)A spark ignites a mixture of propane and air.
A) I only
B) I and II only
C) I and III only
D) II and III only
E) I, II, and III are all spontaneous.
I, II, and III are all spontaneous.
3
In a spontaneous process, the entropy of the universe ___________
A) increases always.
B) decreases always.
C) does not change.
D) may decrease if the entropy of the system decreases sufficiently.
E) may decrease if the entropy of the system increases sufficiently.
A) increases always.
B) decreases always.
C) does not change.
D) may decrease if the entropy of the system decreases sufficiently.
E) may decrease if the entropy of the system increases sufficiently.
increases always.
4
When you increase the volume of a gas, the energy separation between microstates __________
A) increases.
B) decreases.
C) remains unchanged.
D) becomes infinite.
E) disappears.
A) increases.
B) decreases.
C) remains unchanged.
D) becomes infinite.
E) disappears.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
Of the three modes of molecular motion-vibration, rotation, and translation-which requires the greatest amount of energy to cause an excitation from the ground state to the first excited state?
A) vibration
B) rotation
C) translation
D) They all require the same amount of energy.
E) None, as quantized energy states do not apply to these motions.
A) vibration
B) rotation
C) translation
D) They all require the same amount of energy.
E) None, as quantized energy states do not apply to these motions.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
In a spontaneous process, which of the following always increases?
A) the entropy of the system
B) the entropy of the surroundings
C) the entropy of the universe
D) the entropy of the system and the universe
E) the entropy of the system, surroundings and the universe
A) the entropy of the system
B) the entropy of the surroundings
C) the entropy of the universe
D) the entropy of the system and the universe
E) the entropy of the system, surroundings and the universe

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
Which, if any, of A through D is not true of entropy? If they are all true, select E.
A) It is a measure of the distribution of energy in a system at a specific temperature.
B) It is a measure of the number of accessible microstates in a pure substance.
C) It is a property of the universe that increases during a spontaneous process.
D) It is a property of a system that may increase or decrease during a spontaneous process.
E) All of the above are true statements.
A) It is a measure of the distribution of energy in a system at a specific temperature.
B) It is a measure of the number of accessible microstates in a pure substance.
C) It is a property of the universe that increases during a spontaneous process.
D) It is a property of a system that may increase or decrease during a spontaneous process.
E) All of the above are true statements.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
The entropy change in a system (Ssys) during a spontaneous process must be __________
A) greater than zero.
B) less than zero.
C) equal to zero.
D) greater than or equal to zero.
E) greater than, less than, or equal to zero.
A) greater than zero.
B) less than zero.
C) equal to zero.
D) greater than or equal to zero.
E) greater than, less than, or equal to zero.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
Boltzmann derived the relationship, S = k ln W where W is the __________
A) number of microstates.
B) vibrational energy.
C) kinetic energy.
D) Wentworth factor.
E) potential energy.
A) number of microstates.
B) vibrational energy.
C) kinetic energy.
D) Wentworth factor.
E) potential energy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
Heat transfer from the system to the surroundings has a large effect on Ssurr ___________
A) when the temperature of the surroundings is low.
B) when the temperature of the surroundings is high.
C) when the temperature of the system is low.
D) when the temperature of the system is high.
E) at any temperature as the amount of heat transferred is independent of temperature.
A) when the temperature of the surroundings is low.
B) when the temperature of the surroundings is high.
C) when the temperature of the system is low.
D) when the temperature of the system is high.
E) at any temperature as the amount of heat transferred is independent of temperature.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
During a spontaneous chemical reaction, it is found that "Ssys" < 0. This means __________
A) "Ssurr" < 0 and its magnitude is < "Ssys".
B) "Ssurr" < 0 and its magnitude is > "Ssys".
C) "Ssurr" > 0 and its magnitude is < "Ssys".
D) "Ssurr" > 0 and its magnitude is > "Ssys".
E) an error has been made, as Ssys > 0 by necessity for a spontaneous process.
A) "Ssurr" < 0 and its magnitude is < "Ssys".
B) "Ssurr" < 0 and its magnitude is > "Ssys".
C) "Ssurr" > 0 and its magnitude is < "Ssys".
D) "Ssurr" > 0 and its magnitude is > "Ssys".
E) an error has been made, as Ssys > 0 by necessity for a spontaneous process.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
The entropy change of the surroundings, Ssurr, is related to heat transfer q with respect to the system and temperature T by __________
A) -q/Tsys = Ssurr.
B) +q/Tsys = Ssurr.
C) -q/Tsurr = Ssurr.
D) q/Tsurr = Ssurr.
E) None of these, unless the system undergoes a reversible process.
A) -q/Tsys = Ssurr.
B) +q/Tsys = Ssurr.
C) -q/Tsurr = Ssurr.
D) q/Tsurr = Ssurr.
E) None of these, unless the system undergoes a reversible process.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
What types of motion does a molecule of chlorine have?
A) translational, rotational, vibrational
B) kinetic energy and potential energy
C) microstate and macrostate
D) Boltzmann and non-Boltzmann
E) all of the above
A) translational, rotational, vibrational
B) kinetic energy and potential energy
C) microstate and macrostate
D) Boltzmann and non-Boltzmann
E) all of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following states of motion have the highest number of occupied excited states at room temperature-rotational, vibrational, or translational?
A) rotational
B) vibrational
C) translational
D) The number of occupied excited states is the same for the three modes of motion.
E) None of these, as excited states do not apply to these motions.
A) rotational
B) vibrational
C) translational
D) The number of occupied excited states is the same for the three modes of motion.
E) None of these, as excited states do not apply to these motions.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
An oxygen molecule can have several different modes of motions. One type of motion is __________
A) kinetic energy.
B) potential energy.
C) vibrational.
D) elastic.
E) Boltzmann.
A) kinetic energy.
B) potential energy.
C) vibrational.
D) elastic.
E) Boltzmann.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following must be true for the microstates of a system?
(I)The energy of each microstate equals the energy of the system.
(II)The entropy of each microstate equals the entropy of the system.
(III)The number of microstates equals the entropy of the system.
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.
(I)The energy of each microstate equals the energy of the system.
(II)The entropy of each microstate equals the entropy of the system.
(III)The number of microstates equals the entropy of the system.
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following processes are reversible in the thermodynamic sense?
(I) Iron in the open air rusts.
(II) NaCl is dissolved in water and then recovered by the evaporation of the water.
(III) The ice in a mixture of ice and water at 0°C and 1 atm melts.
A) I only
B) II only
C) III only
D) II and III only
E) I, II and III are all reversible.
(I) Iron in the open air rusts.
(II) NaCl is dissolved in water and then recovered by the evaporation of the water.
(III) The ice in a mixture of ice and water at 0°C and 1 atm melts.
A) I only
B) II only
C) III only
D) II and III only
E) I, II and III are all reversible.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
According to the second law of thermodynamics, the change in the entropy of the universe (Suniv) during a spontaneous reaction is __________
A) zero.
B) negative.
C) positive.
D) less than the change in entropy of the system (Ssys).
E) greater than the change in entropy of the system (Ssys).
A) zero.
B) negative.
C) positive.
D) less than the change in entropy of the system (Ssys).
E) greater than the change in entropy of the system (Ssys).

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following must be true for a spontaneous exothermic process?
A) only that Ssys < 0
B) only that Ssys > 0
C) both Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
D) both Ssys < 0 and the magnitude of Ssys > the magnitude of Ssurr
E) either Ssys > 0 or Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
A) only that Ssys < 0
B) only that Ssys > 0
C) both Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr
D) both Ssys < 0 and the magnitude of Ssys > the magnitude of Ssurr
E) either Ssys > 0 or Ssys < 0 and the magnitude of Ssys < the magnitude of Ssurr

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
The gas above the liquid in a sealed bottle of soda is primarily carbon dioxide. Carbon dioxide is also dissolved in the soda. When the distribution of carbon dioxide between the gas and liquid is at equilibrium, molecules of carbon dioxide in the gas phase can still dissolve in the liquid phase if they strike the surface and are captured. Similarly molecules of carbon dioxide can escape from the liquid phase. What is the entropy change of the universe, "Suniv", for the dissolution of carbon dioxide under these conditions?
A) "Suniv" < 0 because the dissolved carbon dioxide has fewer accessible states.
B)" Suniv" > 0 because the dissolved carbon dioxide has fewer accessible states.
C) "Suniv" = 0 because this is an equilibrium situation.
D) "Suniv" < 0 because the gas dissolves spontaneously.
E) "Suniv" > 0 because the gas dissolves spontaneously.
A) "Suniv" < 0 because the dissolved carbon dioxide has fewer accessible states.
B)" Suniv" > 0 because the dissolved carbon dioxide has fewer accessible states.
C) "Suniv" = 0 because this is an equilibrium situation.
D) "Suniv" < 0 because the gas dissolves spontaneously.
E) "Suniv" > 0 because the gas dissolves spontaneously.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
At 0 K, the entropy of a perfect crystal is ___________
A) > 0.
B) = 0.
C) < 0.
D) > 0, = 0, or < 0, depending on the chemical structure of the crystal.
E) > 0 or = 0, depending on the chemical structure of the crystal.
A) > 0.
B) = 0.
C) < 0.
D) > 0, = 0, or < 0, depending on the chemical structure of the crystal.
E) > 0 or = 0, depending on the chemical structure of the crystal.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following will have the greatest standard molar entropy (S°)?
A) NH3(g)
B) He(g)
C) C(s, graphite)
D) H2O( )
)
E) CaCO3(s)
A) NH3(g)
B) He(g)
C) C(s, graphite)
D) H2O(
 )
)E) CaCO3(s)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
Care must be taken when dissolving solid pellets of sodium hydroxide (NaOH) in water as the temperature of the water can rise dramatically. Taking NaOH as the system, what can you deduce about the signs of the entropy change of the system (Ssys) and surroundings (Ssurr) from this?
A) "Ssys" < 0 and "Ssurr" < 0
B)" Ssys" < 0 and "Ssurr" > 0
C) "Ssys" > 0 and "Ssurr" < 0
D)" Ssys" > 0 and "Ssurr" > 0
E) Nothing can be deduced from this limited information.
A) "Ssys" < 0 and "Ssurr" < 0
B)" Ssys" < 0 and "Ssurr" > 0
C) "Ssys" > 0 and "Ssurr" < 0
D)" Ssys" > 0 and "Ssurr" > 0
E) Nothing can be deduced from this limited information.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
Indicate which of the following has the highest entropy at 298 K.
A) 0.5 g of HCN
B) 1 mol of HCN
C) 2 kg of HCN
D) 2 mol of HCN
E) All of the above have the same entropy at 298 K.
A) 0.5 g of HCN
B) 1 mol of HCN
C) 2 kg of HCN
D) 2 mol of HCN
E) All of the above have the same entropy at 298 K.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the relationships A through D between the free energy change of a system and associated entropy changes is true?
A) "Gsys" = +T "Ssystem"
B) "Gsys" = -T "Ssystem"
C) "Gsys" = +T "Suniverse"
D) "Gsys" = -T "Ssurroundings"
E) "Gsys" = -T "Suniverse"
A) "Gsys" = +T "Ssystem"
B) "Gsys" = -T "Ssystem"
C) "Gsys" = +T "Suniverse"
D) "Gsys" = -T "Ssurroundings"
E) "Gsys" = -T "Suniverse"

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
Consider a closed container containing a 1 M solution of HCl above which is air that contains water vapor at its equilibrium vapor pressure. Assume the pressure of the air and water vapor is 1 bar and the temperature of the system is 298 K. Which of the following are in their thermodynamic standard state?
(I)The liquid water
(II)The HCl solution
(III)The water vapor
A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III are all in their standard state.
(I)The liquid water
(II)The HCl solution
(III)The water vapor
A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III are all in their standard state.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
Perfect crystals of carbon monoxide (CO) are difficult to prepare because the very small dipole moment allows a few molecules to align in a pattern like CO OC CO instead of CO CO CO. If such crystals were cooled to 0 K, what would be the value of their absolute entropy?
A) > 0
B) = 0
C) < 0
D) > 0, = 0, or < 0, depending on how carefully it was cooled
E) > 0 or = 0, depending on how carefully it was cooled
A) > 0
B) = 0
C) < 0
D) > 0, = 0, or < 0, depending on how carefully it was cooled
E) > 0 or = 0, depending on how carefully it was cooled

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the processes A-D will lead to a positive change in the entropy of the system? If all of these processes lead to a positive change in the entropy of the system, select E.
A) Sodium chloride crystals form as saltwater evaporates.
B) Helium gas escapes from the hole in a balloon.
C) Stalactites form in a cave.
D) Water freezes in a freezer.
E) All of these lead to a positive change in entropy of the system, as they are all spontaneous.
A) Sodium chloride crystals form as saltwater evaporates.
B) Helium gas escapes from the hole in a balloon.
C) Stalactites form in a cave.
D) Water freezes in a freezer.
E) All of these lead to a positive change in entropy of the system, as they are all spontaneous.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following processes will lead to a decrease in the entropy of the system?
A) Salt crystals dissolve in water.
B) Air escapes from a hole in a balloon.
C) Iron and oxygen react to form rust.
D) Ice melts in your hand.
E) None of these lead to a negative change in the entropy of the system, as they are all spontaneous.
A) Salt crystals dissolve in water.
B) Air escapes from a hole in a balloon.
C) Iron and oxygen react to form rust.
D) Ice melts in your hand.
E) None of these lead to a negative change in the entropy of the system, as they are all spontaneous.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
The standard molar entropy of lead(II) bromide (PbBr2) is 161 J/mol · K. What is the entropy of 2.45 g of PbBr2?
A) +1.07 J/K
B) -1.07 J/K
C) +161 J/K
D) -161 J/K
E) 0 J/K
A) +1.07 J/K
B) -1.07 J/K
C) +161 J/K
D) -161 J/K
E) 0 J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
The following figures represent distributions of two types of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
A)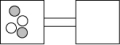
B)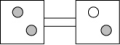
C)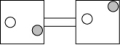
D)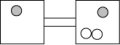
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
The entropy of a NaCl crystal is __________
A) an intensive property and a state function.
B) an intensive property and a path function.
C) an extensive property and a state function.
D) an extensive property and a path function.
E) not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.
A) an intensive property and a state function.
B) an intensive property and a path function.
C) an extensive property and a state function.
D) an extensive property and a path function.
E) not appropriately described in terms of an intensive property, an extensive property, a state function, or a path function.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
Indicate which of the following has the lowest standard molar entropy (S°).
A) CH4(g)
B) CH3CH2OH( )
)
C) H2O(s)
D) Na(s)
E) He(g)
A) CH4(g)
B) CH3CH2OH(
 )
)C) H2O(s)
D) Na(s)
E) He(g)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
The following figures represent distributions of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
A)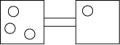
B)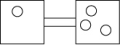
C)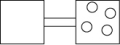
D)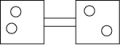
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
Indicate which one of the following reactions result in a positive Ssys.
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O(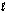 ) HCl(aq)
) HCl(aq)
C) H2(g) + I2(g) 2 Hl(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O(l)
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O(
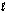 ) HCl(aq)
) HCl(aq)C) H2(g) + I2(g) 2 Hl(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O(l)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
What is the entropy change to the surroundings when 1 mol of ice melts in someone's hand if the hand temperature is 32°C? Assume a final temperature for the water of 0°C. The heat of fusion of ice is 6.01 kJ/mol.
A) -188 J/K
B) -22.0 J/K
C) -19.7 J/K
D) +19.7 J/K
E) +188 J/K
A) -188 J/K
B) -22.0 J/K
C) -19.7 J/K
D) +19.7 J/K
E) +188 J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following graphs best depicts the entropy of a pure substance as the temperature is raised from its solid form through its liquid and gaseous forms?
A)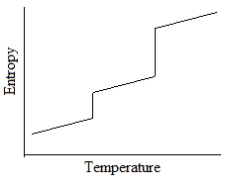
B)
C)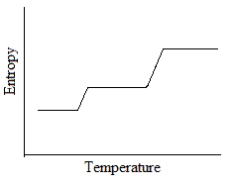
D)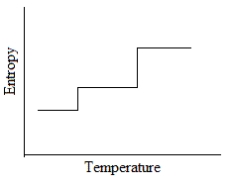
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
If 1 mol of ice melts at its melting point of 273 K, the entropy change for the ice is 22.0 J/K. If the ice melts in someone's hand at 34°C, what is the change in the entropy of the universe? Assume a final temperature for the water of 0°C. The enthalpy of fusion for ice is 6.01 kJ/mol.
A) +19.6 J/K
B) -19.6 J/K
C) +2.4 J/K
D) -2.4 J/K
E) +41.5 J/K
A) +19.6 J/K
B) -19.6 J/K
C) +2.4 J/K
D) -2.4 J/K
E) +41.5 J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
Indicate which one of the following reactions results in a negative Ssys.
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 · 5H2O(s) CuSO4(s) + 5H2O(g)
D) 14O2(g) + 3NH4NO3(s) + C10H22(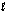 ) 3N2(g) + 17H2O(g) + 10CO2(g)
) 3N2(g) + 17H2O(g) + 10CO2(g)
E) CO2(aq) CO2(g)
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 · 5H2O(s) CuSO4(s) + 5H2O(g)
D) 14O2(g) + 3NH4NO3(s) + C10H22(
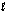 ) 3N2(g) + 17H2O(g) + 10CO2(g)
) 3N2(g) + 17H2O(g) + 10CO2(g)E) CO2(aq) CO2(g)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is in the correct order of standard state entropy?
(I)Diamond < graphite
(II)Liquid water < solid water
(III)NH3 < H2
A) I only
B) II only
C) III only
D) I and II only
E) I and III only
(I)Diamond < graphite
(II)Liquid water < solid water
(III)NH3 < H2
A) I only
B) II only
C) III only
D) I and II only
E) I and III only

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
A reaction with a low enthalpy of reaction value is not spontaneous at low temperature but becomes spontaneous at high temperature. What are the signs for H° and S°, respectively?
A) +, -
B) -, -
C) -, +
D) +, +
E) Insufficient data is provided to answer this question.
A) +, -
B) -, -
C) -, +
D) +, +
E) Insufficient data is provided to answer this question.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
Processes are always spontaneous when __________ (H and S refer to the system).
A) "H" > 0 and "S" < 0
B)"H" < 0 and "S" < 0
C) "H" > 0 and "S" > 0
D) "H" < 0 and "S" > 0
E) None of these is true, as temperature must always be taken into account.
A) "H" > 0 and "S" < 0
B)"H" < 0 and "S" < 0
C) "H" > 0 and "S" > 0
D) "H" < 0 and "S" > 0
E) None of these is true, as temperature must always be taken into account.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
At constant T and P, any reaction will be spontaneous if __________
A) 'Gsys' > 0
B) 'Gsys' < 0
C) 'Ssys" > 0
D)' Ssys" < 0
E) "Hsys' < 0
A) 'Gsys' > 0
B) 'Gsys' < 0
C) 'Ssys" > 0
D)' Ssys" < 0
E) "Hsys' < 0

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
If a reaction A B has G 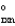 > 0, then when the reaction is at equilibrium there will be __________
> 0, then when the reaction is at equilibrium there will be __________
A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.
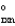 > 0, then when the reaction is at equilibrium there will be __________
> 0, then when the reaction is at equilibrium there will be __________A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
At what temperature does the Fe(s) Fe(g) phase transition occur? H = 415.5 kJ/mol; S = 153.4 J/mol · K.
A) 2162°F
B) 2435°F
C) 4352°F
D) 4416°F
E) 2709°F
A) 2162°F
B) 2435°F
C) 4352°F
D) 4416°F
E) 2709°F

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water. If H° = -56.13 kJ/mol and S° = 79.11 J/mol · K, what is G° for this reaction at 20°C?
A) -79.31 kJ/mol
B) -77.73 kJ/mol
C) -2.324 104 kJ/mol
D) 79.31 kJ/mol
E) -1638 kJ/mol
A) -79.31 kJ/mol
B) -77.73 kJ/mol
C) -2.324 104 kJ/mol
D) 79.31 kJ/mol
E) -1638 kJ/mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
A reaction is at equilibrium at a given temperature and constant pressure when __________
A) "Srxn" = 0.
B) "S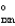 " = 0.
" = 0.
C) "Grxn = 0.
D)" G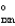 " = 0.
" = 0.
E) "Hrxn" = 0.
A) "Srxn" = 0.
B) "S
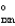 " = 0.
" = 0.C) "Grxn = 0.
D)" G
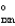 " = 0.
" = 0.E) "Hrxn" = 0.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
The symbol G  (CH4, g) refers to which of the following reactions?
(CH4, g) refers to which of the following reactions?
A) CH4(l) CH4(g)
B) CH4(g) CH4(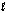 )
)
C) CH4(g) C(g) + 4H(g)
D) C(g) + 4H(g) CH4(g)
E) C(s, graphite) + 2H2(g) CH4(g)
 (CH4, g) refers to which of the following reactions?
(CH4, g) refers to which of the following reactions?A) CH4(l) CH4(g)
B) CH4(g) CH4(
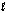 )
)C) CH4(g) C(g) + 4H(g)
D) C(g) + 4H(g) CH4(g)
E) C(s, graphite) + 2H2(g) CH4(g)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
At body temperature, many proteins have a well-defined structure that is essential to their function. As the temperature is raised, however, the structure changes and the protein is no longer functional. This is referred to as protein denaturation. What can be deduced from this information about the signs of the enthalpy and entropy changes for denaturation?
A) "H" > 0 and "S" > 0
B) "H" > 0 and "S" < 0
C) "H" < 0 and "S" > 0
D) "H" < 0 and "S" < 0
E) There is insufficient information to deduce anything about the signs of H and S.
A) "H" > 0 and "S" > 0
B) "H" > 0 and "S" < 0
C) "H" < 0 and "S" > 0
D) "H" < 0 and "S" < 0
E) There is insufficient information to deduce anything about the signs of H and S.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements about equilibrium are true?
(I)Gsys = 0
(II)Ssys = 0
(III)Suniverse = 0
A) I only
B) II only
C) III only
D) Both I and II
E) Both I and III
(I)Gsys = 0
(II)Ssys = 0
(III)Suniverse = 0
A) I only
B) II only
C) III only
D) Both I and II
E) Both I and III

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
The equilibrium vapor pressure for benzene is 94.4 mm Hg. When liquid benzene is in equilibrium with its vapor, we must have __________
A) "G" = 0 and "G°" = 0
B) "G" = 0 and "G°" > 0
C) "G" = 0 and "G°" < 0
D) "G" > 0 and "G°" = 0
E) "G" < 0 and "G°" = 0
A) "G" = 0 and "G°" = 0
B) "G" = 0 and "G°" > 0
C) "G" = 0 and "G°" < 0
D) "G" > 0 and "G°" = 0
E) "G" < 0 and "G°" = 0

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
The entropy of vaporization of water is 109.0 J/mol · K. What is the enthalpy of vaporization of water at its normal boiling point?
A) +10.90 kJ/mol
B) -40.66 kJ/mol
C) +3.42 kJ/mol
D) +40.66 kJ/mol
E) -10.90 kJ/mol
A) +10.90 kJ/mol
B) -40.66 kJ/mol
C) +3.42 kJ/mol
D) +40.66 kJ/mol
E) -10.90 kJ/mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
Dinitrogen tetroxide (N2O4) decomposes to nitrogen dioxide (NO2). If H° = 58.02 kJ/mol and S° = 176.1 J/mol · K, at what temperature are reactants and products in their standard states at equilibrium?
A) +56.5°C
B) +329.5°C
C) -272.7°C
D) +25.0°C
E) +98.3°C
A) +56.5°C
B) +329.5°C
C) -272.7°C
D) +25.0°C
E) +98.3°C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction 3H2(g) + N2(g) 2NH3(g)
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/mol · K. Determine G° at 25°C.
A) +5.897 104 kJ/mol
B) +297.8 kJ/mol
C) -33.32 kJ/mol
D) -16.66 kJ/mol
E) +49.5 kJ/mol
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/mol · K. Determine G° at 25°C.
A) +5.897 104 kJ/mol
B) +297.8 kJ/mol
C) -33.32 kJ/mol
D) -16.66 kJ/mol
E) +49.5 kJ/mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following are true for a reversible process at equilibrium?
(I)Suniv = 0
(II)Ssys = 0
(III)Gsys = 0
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.
(I)Suniv = 0
(II)Ssys = 0
(III)Gsys = 0
A) I only
B) II only
C) III only
D) I and III only
E) I, II, and III are all true.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
Benzene is a liquid under standard conditions. The standard free-energy change for the evaporation of liquid benzene to form benzene vapor is +5.2 kJ/mol. If liquid benzene is placed in an open container under standard conditions, __________
A) it will not evaporate.
B) it will partially evaporate with the equilibrium vapor pressure being less than 1 bar.
C) it will partially evaporate with the equilibrium vapor pressure being equal to 1 bar.
D) it will partially evaporate with the equilibrium vapor pressure being greater than 1 bar.
E) it will completely evaporate.
A) it will not evaporate.
B) it will partially evaporate with the equilibrium vapor pressure being less than 1 bar.
C) it will partially evaporate with the equilibrium vapor pressure being equal to 1 bar.
D) it will partially evaporate with the equilibrium vapor pressure being greater than 1 bar.
E) it will completely evaporate.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
The enthalpy and entropy of vaporization of ethanol are 38.6 kJ/mol and 109.8 J/mol · K, respectively. What is the boiling point of ethanol, in °C?
A) 352°C
B) 78.5°C
C) 2.84°C
D) 624°C
E) Not enough information is given to answer the question.
A) 352°C
B) 78.5°C
C) 2.84°C
D) 624°C
E) Not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
The dissolution of ammonium nitrate in water is a spontaneous endothermic process. It is spontaneous because the system undergoes __________
A) a decrease in enthalpy.
B) an increase in entropy.
C) an increase in enthalpy.
D) a decrease in entropy.
E) an increase in free energy.
A) a decrease in enthalpy.
B) an increase in entropy.
C) an increase in enthalpy.
D) a decrease in entropy.
E) an increase in free energy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Determine Grxn for C4H10( 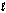 ) +
) + 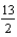 O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
G (J/mol · K)
(J/mol · K)
C4H10(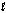 )
)
-15)0
CO2(g)
-394.4
H2O(g)
-228.57
A) -2705 kJ/mol
B) -608.0 kJ/mol
C) -1791 kJ/mol
D) -3457 kJ/mol
E) +608.0 kJ/mol
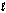 ) +
) + 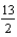 O2(g) 4CO2(g) + 5H2O(g) given the following. Substance
O2(g) 4CO2(g) + 5H2O(g) given the following. SubstanceG
 (J/mol · K)
(J/mol · K)C4H10(
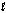 )
)-15)0
CO2(g)
-394.4
H2O(g)
-228.57
A) -2705 kJ/mol
B) -608.0 kJ/mol
C) -1791 kJ/mol
D) -3457 kJ/mol
E) +608.0 kJ/mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
The enthalpy of fusion for benzene is 127.40 kJ/kg, and its melting point is 5.5°C. What is the entropy change when 1 mole of benzene melts at 5.5°C?
A) 9.95 kJ/K
B) 35.7 J/K
C) 1809 J/K
D) 1.81 J/K
E) 127.40 kJ/K
A) 9.95 kJ/K
B) 35.7 J/K
C) 1809 J/K
D) 1.81 J/K
E) 127.40 kJ/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
The standard entropy of diamond is 2.4 J/mol · K. Calculate the entropy per carbon atom in diamond and the number of microstates for each atom. Discuss the number of microstates in terms of the molecular motions accessible to each carbon atom.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
The standard molar enthalpy of fusion for xenon is 2.30 kJ mol-1. Calculate the standard molar entropy for the freezing of liquid xenon given that its normal freezing point is -112°C.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
The standard entropy of N2(g) is 191.5 J/mol · K. Calculate the entropy per nitrogen molecule and the number of microstates for each molecule. Discuss the number of microstates in terms of the molecular motions accessible to each molecule.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
The entropy of fusion for ice at 0°C and 1 atm is 22 J/mol · K. How many joules of heat are required to melt a typical ice cube at 0°C and 1 atm? Assume an ice cube is about 1 oz or 28
g.
g.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
The heat of fusion for water is 333.6 J/g. What is the entropy change for the universe when 1.00 L of water at 0°C freezes at +5°C? Comment on the sign.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
What is a microstate and how are microstates quantitatively related to entropy?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
Determine the normal melting point of benzoic acid in degrees centigrade given the following data.
H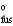 = 18.02 kJ/mol
= 18.02 kJ/mol
S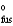 = 45.56 J/mol · K
= 45.56 J/mol · K
H
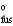 = 18.02 kJ/mol
= 18.02 kJ/molS
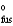 = 45.56 J/mol · K
= 45.56 J/mol · K
Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
Carbon monoxide has a very weak dipole moment. In the formation of solid carbon monoxide some molecules will be reversed in their orientation from C  O - - - C
O - - - C  O to C
O to C  O - - - O
O - - - O  C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
 O - - - C
O - - - C  O to C
O to C  O - - - O
O - - - O  C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
Carbon atoms can be found in a variety of elemental forms. At sufficiently high temperature, carbon is an atomic gas. At room temperature, both tetrahedral networks in diamond and trigonal planar networks in graphite can be found. Trigonal planar networks of carbon atoms are also found in Buckminsterfullerene (C60). How does the standard entropy of these forms vary per mole of carbon?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
The change in standard molar entropy, S for the following reaction is 494.6 J/mol · K at 25C.
2KClO3(s) 2KCl(s) + 3O2(g)
What is the standard molar entropy of O2(g) at 25C?
S°(KClO3, s)= 143.1 J/mol · K
S°(KCl, s)= 82.6 J/mol · K
2KClO3(s) 2KCl(s) + 3O2(g)
What is the standard molar entropy of O2(g) at 25C?
S°(KClO3, s)= 143.1 J/mol · K
S°(KCl, s)= 82.6 J/mol · K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
Only one substance has a standard entropy of 0 J/K, and that is H+(aq). Why is this so?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
Considering the tabulated values for the thermodynamic properties of oxygen gas, why is the standard entropy nonzero but the standard enthalpy and free energy of formation are zero?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
What are the signs of H°, S°, and G° for the conversion of liquid water to ice at 10°C and 1 atm? Briefly explain each answer.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
Draw a graph of entropy versus temperature for a typical substance. Be sure to clearly label phases and phase transitions on the graph.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
Give an example of a spontaneous and a nonspontaneous chemical process and the sign of the entropy change for the universe for each.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
The heat of fusion for water is 333.6 J/g. What is the entropy change for the universe when 1.00 L of water at 0°C freezes at -5°C? Comment on the sign.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
What is the overall standard free-energy change for the following two reactions in terms of G1 and G2?
A + B 2C G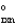 = G1
= G1
C + D E G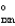 = G2
= G2
A + B 2C G
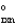 = G1
= G1C + D E G
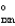 = G2
= G2
Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
Hydrogen iodide can theoretically be made by the reaction of hydrogen gas and iodine by the following reaction.
H2(g) + I2(s) 2HI(g)
What temperature conditions are required for the formation of HI to be favored at standard pressure?
S°(H2, g) = 130.6 J/mol · K
S°(I2, s) = 116.1 J/mol · K
S°(HI, g) = 206.6 J/mol · K
H (HI, g) = 26.5 kJ/mol · K
(HI, g) = 26.5 kJ/mol · K
H2(g) + I2(s) 2HI(g)
What temperature conditions are required for the formation of HI to be favored at standard pressure?
S°(H2, g) = 130.6 J/mol · K
S°(I2, s) = 116.1 J/mol · K
S°(HI, g) = 206.6 J/mol · K
H
 (HI, g) = 26.5 kJ/mol · K
(HI, g) = 26.5 kJ/mol · K
Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
Name and state the first three laws of thermodynamics.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


