Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Of the three modes of molecular motion-vibration, rotation, and translation-which requires the greatest amount of energy to cause an excitation from the ground state to the first excited state?
Free
(Multiple Choice)
4.7/5  (41)
(41)
Correct Answer:
A
The entropy of fusion for ice at 0°C and 1 atm is 22 J/mol · K. How many joules of heat are required to melt a typical ice cube at 0°C and 1 atm? Assume an ice cube is about 1 oz or 28
g.
Free
(Short Answer)
4.8/5  (41)
(41)
Correct Answer:
9300 J
Which of the following processes are spontaneous?
(I)Iron in the open air rusts.
(II)Liquid water in a freezer turns to ice.
(III)A spark ignites a mixture of propane and air.
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
E
Which of the following processes are reversible in the thermodynamic sense?
(I) Iron in the open air rusts.
(II) NaCl is dissolved in water and then recovered by the evaporation of the water.
(III) The ice in a mixture of ice and water at 0°C and 1 atm melts.
(Multiple Choice)
4.9/5  (33)
(33)
Draw a graph of entropy versus temperature for a typical substance. Be sure to clearly label phases and phase transitions on the graph.
(Essay)
4.8/5  (40)
(40)
The enthalpy and entropy of vaporization of ethanol are 38.6 kJ/mol and 109.8 J/mol · K, respectively. What is the boiling point of ethanol, in °C?
(Multiple Choice)
4.9/5  (29)
(29)
Hydrogen iodide can theoretically be made by the reaction of hydrogen gas and iodine by the following reaction.
H2(g) + I2(s) 2HI(g)
What temperature conditions are required for the formation of HI to be favored at standard pressure?
S°(H2, g) = 130.6 J/mol · K
S°(I2, s) = 116.1 J/mol · K
S°(HI, g) = 206.6 J/mol · K
H  (HI, g) = 26.5 kJ/mol · K
(HI, g) = 26.5 kJ/mol · K
(Short Answer)
4.9/5  (44)
(44)
What is a microstate and how are microstates quantitatively related to entropy?
(Essay)
4.8/5  (41)
(41)
Only one substance has a standard entropy of 0 J/K, and that is H+(aq). Why is this so?
(Essay)
4.7/5  (39)
(39)
The standard entropy of N2(g) is 191.5 J/mol · K. Calculate the entropy per nitrogen molecule and the number of microstates for each molecule. Discuss the number of microstates in terms of the molecular motions accessible to each molecule.
(Essay)
4.9/5  (33)
(33)
When you increase the volume of a gas, the energy separation between microstates __________
(Multiple Choice)
4.9/5  (43)
(43)
Carbon atoms can be found in a variety of elemental forms. At sufficiently high temperature, carbon is an atomic gas. At room temperature, both tetrahedral networks in diamond and trigonal planar networks in graphite can be found. Trigonal planar networks of carbon atoms are also found in Buckminsterfullerene (C60). How does the standard entropy of these forms vary per mole of carbon?
(Essay)
4.7/5  (43)
(43)
Carbon monoxide has a very weak dipole moment. In the formation of solid carbon monoxide some molecules will be reversed in their orientation from C  O - - - C
O - - - C  O to C
O to C  O - - - O
O - - - O  C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
C. What do you expect the absolute entropy of carbon monoxide solid to be at 0 K, and why?
(Essay)
4.7/5  (28)
(28)
What is the overall standard free-energy change for the following two reactions in terms of G1 and G2?
A + B 2C G 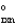 = G1
C + D E G
= G1
C + D E G 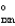 = G2
= G2
(Essay)
4.9/5  (28)
(28)
Boltzmann derived the relationship, S = k ln W where W is the __________
(Multiple Choice)
4.7/5  (46)
(46)
Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction 3H2(g) + N2(g) 2NH3(g)
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/mol · K. Determine G° at 25°C.
(Multiple Choice)
4.8/5  (30)
(30)
At body temperature, many proteins have a well-defined structure that is essential to their function. As the temperature is raised, however, the structure changes and the protein is no longer functional. This is referred to as protein denaturation. What can be deduced from this information about the signs of the enthalpy and entropy changes for denaturation?
(Multiple Choice)
4.8/5  (36)
(36)
Which, if any, of A through D is not true of entropy? If they are all true, select E.
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following must be true for the microstates of a system?
(I)The energy of each microstate equals the energy of the system.
(II)The entropy of each microstate equals the entropy of the system.
(III)The number of microstates equals the entropy of the system.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)