Deck 10: Forces Between Ions and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 10: Forces Between Ions and Molecules
1
Dispersion forces are due to __________
A) permanent dipoles.
B) temporary dipoles.
C) hydrogen bonding.
D) ionic interactions.
E) protons.
A) permanent dipoles.
B) temporary dipoles.
C) hydrogen bonding.
D) ionic interactions.
E) protons.
temporary dipoles.
2
Dipole-dipole interactions typically are not as strong as ion-dipole interactions because __________
A) ion-dipole interactions involve partial charges caused by the equal sharing of electrons by atoms forming a bond.
B) dipole-dipole interactions involve the complete transfer of charge between atoms.
C) ion-dipole interactions only involve the partial transfer of charge between atoms.
D) ion-dipole interactions always involve water.
E) dipole-dipole interactions only involve partial charges caused by unequal sharing of electrons by atoms forming a bond.
A) ion-dipole interactions involve partial charges caused by the equal sharing of electrons by atoms forming a bond.
B) dipole-dipole interactions involve the complete transfer of charge between atoms.
C) ion-dipole interactions only involve the partial transfer of charge between atoms.
D) ion-dipole interactions always involve water.
E) dipole-dipole interactions only involve partial charges caused by unequal sharing of electrons by atoms forming a bond.
dipole-dipole interactions only involve partial charges caused by unequal sharing of electrons by atoms forming a bond.
3
Which of the following compounds is capable of hydrogen bonding?
A) CH3OCH3
B) CH3COCH3
C) CH3CH2OH
D) H2CO
E) CH3F
A) CH3OCH3
B) CH3COCH3
C) CH3CH2OH
D) H2CO
E) CH3F
CH3CH2OH
4
Which of the following solvents will involve ion-dipole interactions with Na+?
A) benzene (C6H6)
B) carbon tetrachloride (CCl4)
C) chloroform (CHCl3)
D) cyclohexane (C6H12)
E) pentane (C5H12)
A) benzene (C6H6)
B) carbon tetrachloride (CCl4)
C) chloroform (CHCl3)
D) cyclohexane (C6H12)
E) pentane (C5H12)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following will require the greatest energy input to separate the ions?
A) MgI2
B) MgF2
C) MgCl2
D) MgBr2
E) NaCl
A) MgI2
B) MgF2
C) MgCl2
D) MgBr2
E) NaCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
For a molecule to exhibit dipole - dipole interactions, it must __________
A) have a temporary dipole moment.
B) have a hydrogen bound to an oxygen, nitrogen, or fluorine.
C) have a permanent dipole moment.
D) be an ion.
E) have 3 or more atoms.
A) have a temporary dipole moment.
B) have a hydrogen bound to an oxygen, nitrogen, or fluorine.
C) have a permanent dipole moment.
D) be an ion.
E) have 3 or more atoms.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
For molecules or atoms with the same mass, which of the following is the weakest intermolecular interaction?
A) ion-dipole
B) hydrogen bonding
C) dipole-dipole
D) dispersion
E) ion-induced dipole
A) ion-dipole
B) hydrogen bonding
C) dipole-dipole
D) dispersion
E) ion-induced dipole

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Ion interaction energies are determined by Coulomb's law: 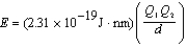 Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
A) -7.36 10-19 J
B) -7.36 10-22 J
C) 7.36 10-19 J
D) 1.74 10-18 J
E) -7.36 10-25 J
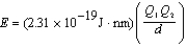 Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.A) -7.36 10-19 J
B) -7.36 10-22 J
C) 7.36 10-19 J
D) 1.74 10-18 J
E) -7.36 10-25 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Coulomb's law states that the energy of attraction between ions depends __________
A) only on the charge of the cation.
B) only on the charge of the anion.
C) directly on the distance between the ions.
D) on the charges of both ions.
E) on the temperature.
A) only on the charge of the cation.
B) only on the charge of the anion.
C) directly on the distance between the ions.
D) on the charges of both ions.
E) on the temperature.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Arrange the three compounds sodium chloride, magnesium chloride, and aluminum chloride in order of increasing melting point.
A) NaCl < MgCl2 < AlCl3
B) MgCl2 < NaCl < AlCl3
C) AlCl3 < MgCl2 < NaCl
D) AlCl3 < NaCl < MgCl2
E) NaCl < AlCl3 < MgCl2
A) NaCl < MgCl2 < AlCl3
B) MgCl2 < NaCl < AlCl3
C) AlCl3 < MgCl2 < NaCl
D) AlCl3 < NaCl < MgCl2
E) NaCl < AlCl3 < MgCl2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Predict which of the following compounds has the highest melting point.
A) NaF
B) KCl
C) RbCl
D) BeF2
E) NaCl
A) NaF
B) KCl
C) RbCl
D) BeF2
E) NaCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following requires the smallest energy to separate the ions?
A) CaF2
B) KF
C) NaF
D) MgF2
E) LiCl
A) CaF2
B) KF
C) NaF
D) MgF2
E) LiCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The interaction energy of LiF is -1.14 10-18 J. What is the distance between the Li+ and F- ions?
A) 203 nm
B) 203 pm
C) 0.203 pm
D) 494 pm
E) 302 pm
A) 203 nm
B) 203 pm
C) 0.203 pm
D) 494 pm
E) 302 pm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Based on their boiling points, which of the following compounds has the largest dipole-dipole interaction?
A) propane (231 K)
B) dimethyl ether (248 K)
C) acetonitrile (355 K)
D) methyl chloride (249 K)
E) butane (135 K)
A) propane (231 K)
B) dimethyl ether (248 K)
C) acetonitrile (355 K)
D) methyl chloride (249 K)
E) butane (135 K)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following will have the largest cation-anion attraction?
A) NaF
B) NaCl
C) NaBr
D) NaI
E) CsCl
A) NaF
B) NaCl
C) NaBr
D) NaI
E) CsCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following polar compounds is likely to have the highest boiling point?
A) CH3OCH3
B) CH3CH2OH
C) (CH3)2CO
D) H2CO
E) CO
A) CH3OCH3
B) CH3CH2OH
C) (CH3)2CO
D) H2CO
E) CO

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is capable of dipole-dipole interactions?
A) CH4
B) CO2
C) H2CO
D) SF6
E) NH4+
A) CH4
B) CO2
C) H2CO
D) SF6
E) NH4+

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Polarizability refers to __________
A) the ease with which the electron cloud of an atom or molecule can be distorted.
B) the magnitude of the dipole moment of a molecule.
C) the ease with which a hydrogen bond can form.
D) the perturbation in electron density because of hydrogen bonding.
E) the ability to transmit polarized light.
A) the ease with which the electron cloud of an atom or molecule can be distorted.
B) the magnitude of the dipole moment of a molecule.
C) the ease with which a hydrogen bond can form.
D) the perturbation in electron density because of hydrogen bonding.
E) the ability to transmit polarized light.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
A)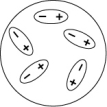
B)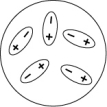
C)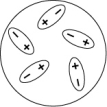
D)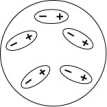
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
Ion-dipole forces always require
A) an ion and a water molecule.
B) a cation and a water molecule.
C) an anion and a polar molecule.
D) an ion and a polar molecule.
E) a polar and a nonpolar molecule.
A) an ion and a water molecule.
B) a cation and a water molecule.
C) an anion and a polar molecule.
D) an ion and a polar molecule.
E) a polar and a nonpolar molecule.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
The solubility of a compound is determined by __________
A) solute-solvent interactions.
B) solute-solute interactions.
C) solvent-solvent interactions.
D) the change in entropy.
E) all of the items listed
A) solute-solvent interactions.
B) solute-solute interactions.
C) solvent-solvent interactions.
D) the change in entropy.
E) all of the items listed

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Given the van der Waals a constant values for the following gases, which gas has the lowest boiling point?
A) N2 a = 1.35 L2 · atm/mol2
B) O2 a = 1.36 L2 · atm/mol2
C) NH3 a = 4.25 L2 · atm/mol2
D) CH4 a = 2.27 L2 · atm/mol2
E) SO2 a = 6.71 L2 · atm/mol2
A) N2 a = 1.35 L2 · atm/mol2
B) O2 a = 1.36 L2 · atm/mol2
C) NH3 a = 4.25 L2 · atm/mol2
D) CH4 a = 2.27 L2 · atm/mol2
E) SO2 a = 6.71 L2 · atm/mol2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following pairs of compounds is most likely to be immiscible?
A) Br2 and C6H6
B) H2O and CH3CH2OH
C) CCl4 and H2CO
D) CH3OH and CH3CH2OH
E) H2O and NH3
A) Br2 and C6H6
B) H2O and CH3CH2OH
C) CCl4 and H2CO
D) CH3OH and CH3CH2OH
E) H2O and NH3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Hydrophilic substances __________
A) are immiscible in water.
B) are insoluble in water.
C) are soluble in water.
D) are hydrated.
E) generally do not hydrogen bond.
A) are immiscible in water.
B) are insoluble in water.
C) are soluble in water.
D) are hydrated.
E) generally do not hydrogen bond.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds would you most appropriately call hydrophobic?
A) CH4
B) H2CO
C) CO
D) HCl
E) NaCl
A) CH4
B) H2CO
C) CO
D) HCl
E) NaCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
When two liquids mix completely in all proportions, they are __________
A) soluble.
B) miscible.
C) insoluble.
D) solvated.
E) ubiquitous.
A) soluble.
B) miscible.
C) insoluble.
D) solvated.
E) ubiquitous.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Indicate which of the following molecules exhibits the greatest dispersion forces.
A) CH3CH3
B) CH3CH2CH3
C) C2H2
D) CH4
E) CH3CH2CH2CH3
A) CH3CH3
B) CH3CH2CH3
C) C2H2
D) CH4
E) CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following gases would you expect to have the largest van der Waals a constant value?
A) Kr
B) N2
C) O2
D) H2
E) Ne
A) Kr
B) N2
C) O2
D) H2
E) Ne

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following compounds would you expect to be a solid at room temperature and atmospheric pressure?
A) C2H4 (an alkene)
B) HCl (an acid)
C) CH3CH2CH2CH3 (an alkane)
D) C10H8 (an aromatic hydrocarbon)
E) C6H6 (an aromatic hydrocarbon)
A) C2H4 (an alkene)
B) HCl (an acid)
C) CH3CH2CH2CH3 (an alkane)
D) C10H8 (an aromatic hydrocarbon)
E) C6H6 (an aromatic hydrocarbon)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Indicate which of the following compounds will have the lowest boiling point.
A) CCl4
B) CI4
C) CF4
D) CH4
E) CBr4
A) CCl4
B) CI4
C) CF4
D) CH4
E) CBr4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following substances would you expect to have the largest van der Waals a constant value?
A) CO2
B) F2
C) CH3CH2OH
D) CH3Cl
E) N2
A) CO2
B) F2
C) CH3CH2OH
D) CH3Cl
E) N2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Given the van der Waals a constant values for the following gases, which gas has the greatest intermolecular forces?
A) N2 a = 1.35 L2 · atm/mol2
B) O2 a = 1.36 L2 · atm/mol2
C) NH3 a = 4.25 L2 · atm/mol2
D) CH4 a = 2.27 L2 · atm/mol2
E) SO2 a = 6.71 L2 · atm/mol2
A) N2 a = 1.35 L2 · atm/mol2
B) O2 a = 1.36 L2 · atm/mol2
C) NH3 a = 4.25 L2 · atm/mol2
D) CH4 a = 2.27 L2 · atm/mol2
E) SO2 a = 6.71 L2 · atm/mol2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
A substance that is _____ will be insoluble in water, but a substance that is _____ will be soluble in water.
A) hydrophobic; immiscible
B) immiscible; hydrophobic
C) hydrophilic; miscible
D) hydrophobic; hydrophilic
E) miscible; immiscible
A) hydrophobic; immiscible
B) immiscible; hydrophobic
C) hydrophilic; miscible
D) hydrophobic; hydrophilic
E) miscible; immiscible

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds would be most soluble in carbon tetrachloride, CCl4?
A) H2O
B) CH3OH
C) NH3
D) C6H6
E) HCl
A) H2O
B) CH3OH
C) NH3
D) C6H6
E) HCl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds do you expect to be most soluble in water?
A) CO2
B) CCl4
C) O2
D) SiO2
E) NH3
A) CO2
B) CCl4
C) O2
D) SiO2
E) NH3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
Under similar conditions, which of the following gases will behave less like an ideal gas than the others?
A) NH3
B) HCl
C) CH4
D) H2
E) O2
A) NH3
B) HCl
C) CH4
D) H2
E) O2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following representations best shows the formation of an instantaneous dipole moment between two nonpolar molecules?
A)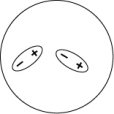
B)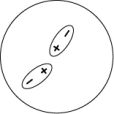
C)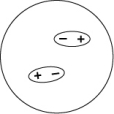
D)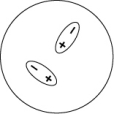
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules will have the highest boiling point?
A) CO2
B) C6H6 (benzene)
C) C6F6 (hexafluorobenzene)
D) C2H2
E) CF4
A) CO2
B) C6H6 (benzene)
C) C6F6 (hexafluorobenzene)
D) C2H2
E) CF4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
Indicate which of the following pairs of compounds is most likely to be miscible.
A) H2O and CH3CH2CH2CH3
B) Br2 and HI
C) HF and CCl4
D) CCl4 and Br2
E) CCl4 and NH3
A) H2O and CH3CH2CH2CH3
B) Br2 and HI
C) HF and CCl4
D) CCl4 and Br2
E) CCl4 and NH3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Molecular nitrogen (N2) interacts with water and is sparingly soluble in water due to _____
A) dispersion forces.
B) dipole-induced dipole forces.
C) ion - dipole forces.
D) hydrogen bonding.
E) dipole - dipole forces.
A) dispersion forces.
B) dipole-induced dipole forces.
C) ion - dipole forces.
D) hydrogen bonding.
E) dipole - dipole forces.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
The resistance of a liquid to an increase in its surface area is __________
A) surface tension.
B) viscosity.
C) capillary action.
D) a meniscus.
E) impossible.
A) surface tension.
B) viscosity.
C) capillary action.
D) a meniscus.
E) impossible.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following substances is a solid at 25°C and 1 atm?
A)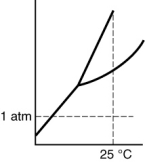
B)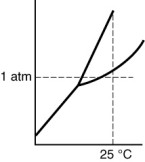
C)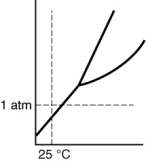
D)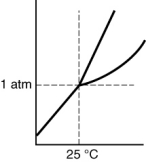
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
The density of water decreases as it is cooled from 4.0°C to freezing because __________
A) water molecules decrease in size as they cool because energy is released.
B) a network of hydrogen bonds between water molecules is formed.
C) water molecules become more rigid as they cool.
D) hydrogen bonds in liquid water are longer than they are in ice.
E) all substances contract just before freezing.
A) water molecules decrease in size as they cool because energy is released.
B) a network of hydrogen bonds between water molecules is formed.
C) water molecules become more rigid as they cool.
D) hydrogen bonds in liquid water are longer than they are in ice.
E) all substances contract just before freezing.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below. Which gas will show the smallest deviation from ideal gas behavior? 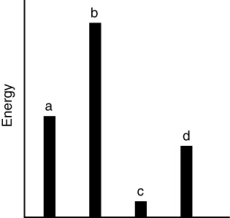
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the lowest boiling point? 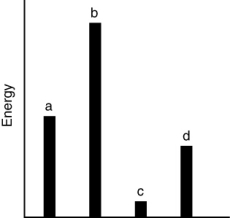
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance is most likely to be a gas at room temperature? 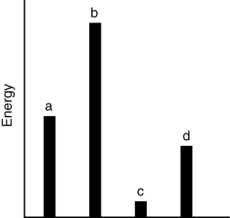
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
Viscosity is a measure of a substance's __________
A) ability to resist changes in its surface area.
B) surface tension.
C) resistance to flow.
D) compressibility.
E) color.
A) ability to resist changes in its surface area.
B) surface tension.
C) resistance to flow.
D) compressibility.
E) color.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the highest melting point? 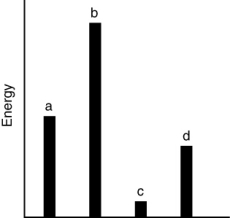
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following substances has a solid whose freezing point will decrease with increasing pressure?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
At the triple point of a substance, __________
A) three phases are present in equilibrium.
B) the solid sublimes.
C) the gas condenses to a liquid.
D) solid and liquid are in equilibrium.
E) only one phase appears to be present.
A) three phases are present in equilibrium.
B) the solid sublimes.
C) the gas condenses to a liquid.
D) solid and liquid are in equilibrium.
E) only one phase appears to be present.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
What does the line indicated in the following phase diagram represent? 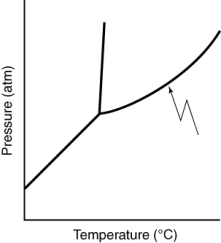
A) s-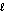 boundary
boundary
B) s-g boundary
C)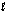 -g boundary
-g boundary
D) triple point
E) s-s boundary

A) s-
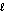 boundary
boundaryB) s-g boundary
C)
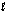 -g boundary
-g boundaryD) triple point
E) s-s boundary

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
In which of the following diagrams are the cohesive interactions between molecules in the liquid greater than the adhesive forces between the liquid and the walls of the tube? 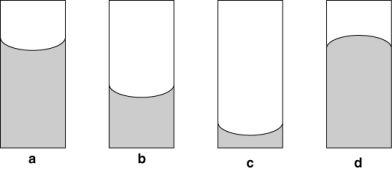
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the highest boiling point? 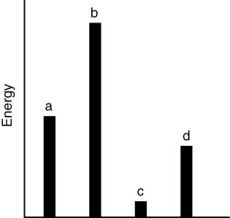
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the lowest melting point? 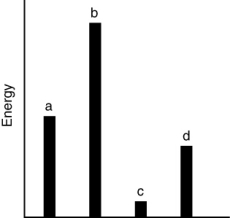
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
At the critical point, __________
A) all the liquid has evaporated.
B) the densities of the solid and liquid are the same.
C) the densities of the solid and the gas are the same.
D) the densities of the gas and the liquid are the same.
E) a critical mass has been reached.
A) all the liquid has evaporated.
B) the densities of the solid and liquid are the same.
C) the densities of the solid and the gas are the same.
D) the densities of the gas and the liquid are the same.
E) a critical mass has been reached.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following substances has a solid that is less dense than the liquid?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
A phase diagram shows the states of a substance as a function of _____ and _____.
A) pressure, volume
B) volume, temperature
C) pressure, temperature
D) concentration, temperature
E) density, pressure
A) pressure, volume
B) volume, temperature
C) pressure, temperature
D) concentration, temperature
E) density, pressure

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
At the point marked with a dot on the phase diagram, the solid will __________ 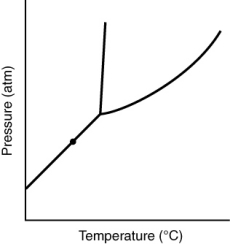
A) be indistinguishable from the gas.
B) boil.
C) melt.
D) sublime.
E) liquify.

A) be indistinguishable from the gas.
B) boil.
C) melt.
D) sublime.
E) liquify.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the phase diagram for a substance shown here. The solid phase is ________ than the liquid phase. 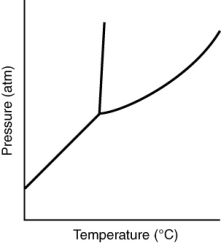
A) more dense
B) less dense
C) more massive
D) less massive
E) hotter

A) more dense
B) less dense
C) more massive
D) less massive
E) hotter

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Water forms a concave meniscus in a glass tube because __________
A) the cohesive forces between water molecules are larger than the adhesive forces between water molecules and the glass wall.
B) the adhesive forces between water molecules and the glass wall are larger than the cohesive forces between water molecules.
C) water molecules form hydrogen bonds with each other.
D) water molecules repel each other.
E) the glass wall is wet.
A) the cohesive forces between water molecules are larger than the adhesive forces between water molecules and the glass wall.
B) the adhesive forces between water molecules and the glass wall are larger than the cohesive forces between water molecules.
C) water molecules form hydrogen bonds with each other.
D) water molecules repel each other.
E) the glass wall is wet.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds so any missing bonds are implicitly bonds to hydrogen atoms.
A)
B)
C)
D)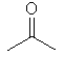
E)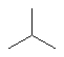
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
The temperature at point b in the phase diagram below is the __________ 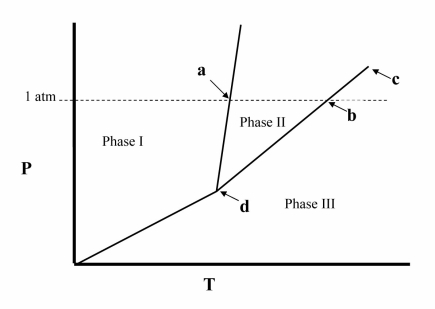
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
A hydration sphere forms around an ion in aqueous solution due to __________
A) ion-dipole interactions.
B) ion-hydrogen-bonding interactions.
C) dispersion forces.
D) dipole-dipole interactions.
E) ion-induced dipole interactions.
A) ion-dipole interactions.
B) ion-hydrogen-bonding interactions.
C) dispersion forces.
D) dipole-dipole interactions.
E) ion-induced dipole interactions.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement about the phase diagram below is not correct? 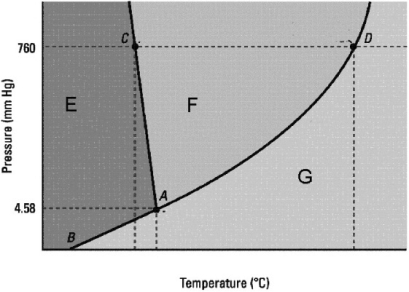
A) The critical point is not shown.
B) D is the normal boiling point.
C) The vapor pressure of the solid is zero below the triple point.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.

A) The critical point is not shown.
B) D is the normal boiling point.
C) The vapor pressure of the solid is zero below the triple point.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance is most likely to be a solid at room temperature? 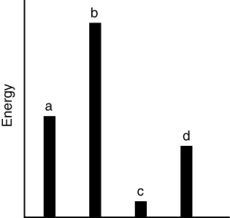
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
Point d in the phase diagram below is the __________ 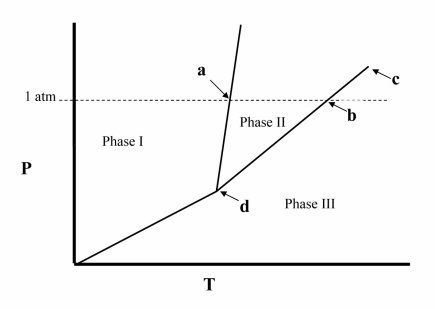
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
The boiling point of HBr is higher than that of HCl because HBr has __________ (The dipole moments are 1.08 D for HCl and 0.82 D for HBr.)
A) fewer electrons.
B) larger dispersion interactions.
C) hydrogen bonding.
D) larger dipole-dipole interactions.
E) more ionic character.
A) fewer electrons.
B) larger dispersion interactions.
C) hydrogen bonding.
D) larger dipole-dipole interactions.
E) more ionic character.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
Rank the following compounds in order of increasing attraction between their ions: KBr, SrBr2, and CsBr.
A) CsBr < KBr < SrBr2
B) SrBr2 < CsBr < KBr
C) KBr < SrBr2 < CsBr
D) CsBr < SrBr2 < KBr
E) SrBr2 < KBr < CsBr
A) CsBr < KBr < SrBr2
B) SrBr2 < CsBr < KBr
C) KBr < SrBr2 < CsBr
D) CsBr < SrBr2 < KBr
E) SrBr2 < KBr < CsBr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the substances a-d in the following figure has the weakest intermolecular forces? 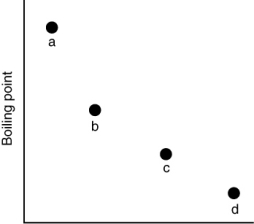
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
When sodium chloride dissolves in water, how do the water molecules orient around the ions?
A) Water molecules are randomly oriented around the ions.
B) The hydrogen atoms point toward both the sodium and the chloride.
C) The oxygen atoms point toward both the sodium and the chloride.
D) Around sodium the hydrogen atoms point toward the sodium, and around chloride the oxygen atoms point toward the chloride.
E) Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride.
A) Water molecules are randomly oriented around the ions.
B) The hydrogen atoms point toward both the sodium and the chloride.
C) The oxygen atoms point toward both the sodium and the chloride.
D) Around sodium the hydrogen atoms point toward the sodium, and around chloride the oxygen atoms point toward the chloride.
E) Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
The temperature at point a in the phase diagram below is the __________ 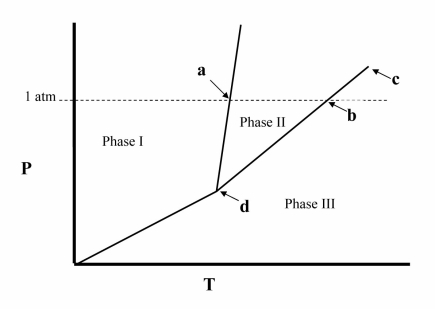
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Which alcohol should be most soluble in a nonpolar solvent such as hexane, C6H14?
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OH
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following compounds will have the strongest dipole-dipole interactions between its molecules?
A) CF4
B) CH4
C) CH3F
D) CH2F2
E) CH3Cl
A) CF4
B) CH4
C) CH3F
D) CH2F2
E) CH3Cl

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
Arrange the following compounds in order of increasing dispersion interactions: CCl4, CH4, C3H8.
A) CH4 < C3H8 < CCl4
B) CCl4 < CH4 < C3H8
C) C3H8 < CH4 < CCl4
D) CCl4 < C3H8 < CH4
E) CH4 < CCl4 < C3H8
A) CH4 < C3H8 < CCl4
B) CCl4 < CH4 < C3H8
C) C3H8 < CH4 < CCl4
D) CCl4 < C3H8 < CH4
E) CH4 < CCl4 < C3H8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following substances would you predict to have the highest boiling point? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds so any missing bonds are implicitly bonds to hydrogen atoms.
A)
B)
C)
D)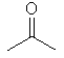
E)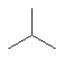
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 10-2 mol/L · atm at body temperature, 37°C. Calculate the molar concentration of oxygen in blood for a scuba diver where the air pressure is 2.0 atm. The mole fraction of oxygen in air is 0.209.
A) 7.8 10-3 M
B) 2.7 10-3 M
C) 1.5 10-2 M
D) 1.3 10-2 M
E) 0.11 M
A) 7.8 10-3 M
B) 2.7 10-3 M
C) 1.5 10-2 M
D) 1.3 10-2 M
E) 0.11 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 10-2 mol/L · atm at body temperature, 37°C. Calculate the molar concentration of oxygen in blood for an alpine climber where the atmospheric pressure is 0.45 atm. The mole fraction of oxygen in air is 0.209.
A) 7.8 10-3 M
B) 3.5 10-3 M
C) 2.3 10-2 M
D) 1.3 10-2 M
E) 0.11 M
A) 7.8 10-3 M
B) 3.5 10-3 M
C) 2.3 10-2 M
D) 1.3 10-2 M
E) 0.11 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
Point c in the phase diagram below is the __________ 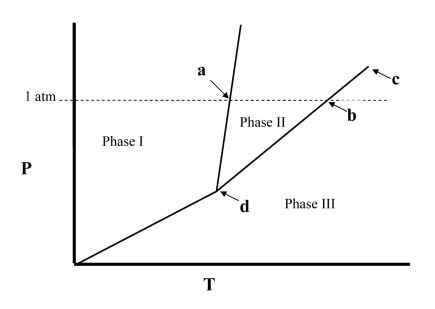
A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

A) critical point.
B) triple point.
C) transition point.
D) normal freezing point.
E) normal boiling point.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
Water contains about 42 mg of oxygen per liter at 20°C, and 1 atm. What is Henry's law constant (mol/L atm) for oxygen dissolving in water?
A) 0.25 mol/L · atm
B) 1.3 10-3 mol/L · atm
C) 1.9 10-2 mol/L · atm
D) 3.7 10-2 mol/L · atm
E) 0.010 mol/L · atm
A) 0.25 mol/L · atm
B) 1.3 10-3 mol/L · atm
C) 1.9 10-2 mol/L · atm
D) 3.7 10-2 mol/L · atm
E) 0.010 mol/L · atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
Which type of intermolecular interaction exists for all compounds?
A) ion-ion
B) dipole-dipole
C) dispersion
D) hydrogen bonding
E) dipole-induced dipole
A) ion-ion
B) dipole-dipole
C) dispersion
D) hydrogen bonding
E) dipole-induced dipole

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


