Exam 10: Forces Between Ions and Molecules
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Water forms a concave meniscus in a glass tube because __________
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
Rank the following compounds in order of increasing attraction between their ions: KBr, SrBr2, and CsBr.
Free
(Multiple Choice)
4.7/5  (46)
(46)
Correct Answer:
A
Which of the following representations best shows the formation of an instantaneous dipole moment between two nonpolar molecules?
Free
(Multiple Choice)
4.8/5  (48)
(48)
Correct Answer:
A
Which of the following compounds do you expect to be most soluble in water?
(Multiple Choice)
4.9/5  (42)
(42)
Which type of intermolecular interaction exists for all compounds?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following substances would you expect to have the largest van der Waals a constant value?
(Multiple Choice)
4.8/5  (31)
(31)
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 10-2 mol/L · atm at body temperature, 37°C. Calculate the molar concentration of oxygen in blood for a scuba diver where the air pressure is 2.0 atm. The mole fraction of oxygen in air is 0.209.
(Multiple Choice)
4.9/5  (37)
(37)
Why do the strengths of dispersion interactions generally increase with the molar mass of the compound?
(Essay)
4.8/5  (29)
(29)
CH2F2 has a dipole moment of 1.93 D and a boiling point of -52°C. CH2Cl2 has a dipole moment of 1.60 D and a boiling point of 40°C. Why is the boiling point of dichloromethane so much higher than that of difluoromethane?
(Essay)
4.8/5  (36)
(36)
Water at 20°C exposed to air at 1.0 atm pressure can contain a maximum of 8.6 mg/L of oxygen. Calculate the value of Henry's law constant (mol/L · atm) for oxygen at this temperature. The mole fraction of oxygen in air is 0.209.
(Essay)
4.8/5  (40)
(40)
Indicate which of the following molecules exhibits the greatest dispersion forces.
(Multiple Choice)
4.7/5  (34)
(34)
The boiling point of HBr is higher than that of HCl because HBr has __________ (The dipole moments are 1.08 D for HCl and 0.82 D for HBr.)
(Multiple Choice)
4.8/5  (42)
(42)
Sketch a phase diagram for water and correctly label all the parts (areas, lines, points, key temperatures).
(Essay)
5.0/5  (38)
(38)
A phase diagram shows the states of a substance as a function of _____ and _____.
(Multiple Choice)
4.8/5  (37)
(37)
For each of the following pairs of compounds, identify the one that is more likely to be soluble in water. Explain the rationale for your choice.
A) Br2 or NaBr
B) CH3CH2OH or CH3OCH3
C) CO2 or KOH
(Essay)
5.0/5  (36)
(36)
Consider the phase diagram for a substance shown here. The solid phase is ________ than the liquid phase. 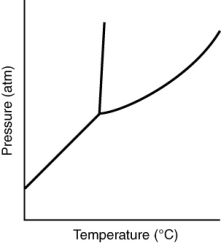
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following will have the largest cation-anion attraction?
(Multiple Choice)
4.8/5  (37)
(37)
Arrange the following compounds in order of increasing attraction between their ions: MgO, CaO, BaO.
(Short Answer)
4.8/5  (44)
(44)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)