Deck 4: Solution Chemistry and the Hydrosphere
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/98
Play
Full screen (f)
Deck 4: Solution Chemistry and the Hydrosphere
1
If there are 0.505 g of NaCl left in a beaker that originally contained 75.0 mL of saltwater, what must have been the concentration of the original solution?
A) 0.00647 M
B) 0.0115 M
C) 0.0673 M
D) 0.115 M
E) 0.673 M
A) 0.00647 M
B) 0.0115 M
C) 0.0673 M
D) 0.115 M
E) 0.673 M
0.115 M
2
Diluting 1.0 mL of a 1.0 M solution to 1000 mL results in a solution that is 0.001 M. Repeating this 1000-fold dilution process five more times results in a concentration of __________
A) 1.0 10-8 M.
B) 5.0 10-3 M.
C) 1.0 10-18 M.
D) 1.0 10-9 M.
E) 2.0 10-4 M.
A) 1.0 10-8 M.
B) 5.0 10-3 M.
C) 1.0 10-18 M.
D) 1.0 10-9 M.
E) 2.0 10-4 M.
1.0 10-18 M.
3
What volume of 12 M HCl solution needs to be diluted to produce 500 mL of 3.0 M HCl solution?
A) 125 mL
B) 250 mL
C) 500 mL
D) 2000 mL
E) 1000 mL
A) 125 mL
B) 250 mL
C) 500 mL
D) 2000 mL
E) 1000 mL
125 mL
4
Which picture best represents an atomic-level view of a nonelectrolyte solution (water molecules not shown)?
A)
B)
C)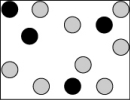
D)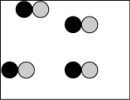
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
5
Determine the molar concentration of an aqueous solution of lead(II) nitrate solution that is 26 ppb (parts per billion) Pb(NO3)2.
A) 7.85 10-14 M
B) 2.60 10-8 M
C) 4.26 10-11 M
D) 7.85 10-8 M
E) 7.85 10-11M
A) 7.85 10-14 M
B) 2.60 10-8 M
C) 4.26 10-11 M
D) 7.85 10-8 M
E) 7.85 10-11M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the molar concentration of ethanol (C2H6O) in a wine that is 14% ethanol by mass. The density of this wine is 0.93 g/cm3.
A) 0.063 M
B) 13.0 M
C) 0.14 M
D) 2.8 M
E) 3.0 M
A) 0.063 M
B) 13.0 M
C) 0.14 M
D) 2.8 M
E) 3.0 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
7
A chemistry student attempted to make a 0.2000 M solution of NaCl using a 100.0 mL volumetric flask. She added exactly 100.0 mL of water to the flask, then added 0.02000 mol of NaCl, and found that the total volume in the flask was above the 100.0 mL mark. What was the concentration of the solution?
A) exactly 0.2000 M
B) a bit less than 0.2000 M
C) a bit more than 0.2000 M
D) exactly 0.2002 M
E) There is insufficient information to select one of the above responses.
A) exactly 0.2000 M
B) a bit less than 0.2000 M
C) a bit more than 0.2000 M
D) exactly 0.2002 M
E) There is insufficient information to select one of the above responses.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
8
In the dilution of 10.0 mL of a 0.10 M solution of HCl to a volume of 20.0 mL, what remains unchanged?
A) the moles of HCl in the solution
B) the concentration of the HCl solution
C) the volume of the HCl solution
D) the mass of the HCl solution
E) All of the above change.
A) the moles of HCl in the solution
B) the concentration of the HCl solution
C) the volume of the HCl solution
D) the mass of the HCl solution
E) All of the above change.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
9
The proof of liquor is defined as the percentage of ethanol it contains times two. If vodka is 80 proof, what is the solvent in vodka?
A) vodka
B) water
C) ethanol
D) not enough information to answer
E) the same as the solute in this case
A) vodka
B) water
C) ethanol
D) not enough information to answer
E) the same as the solute in this case

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
10
How many grams of sodium chloride, NaCl, are needed to make up 1.00 L of a solution that is 2.00 M ?
A) 23.0 g
B) 29.2 g
C) 58.4 g
D) 117 g
E) 35.5 g
A) 23.0 g
B) 29.2 g
C) 58.4 g
D) 117 g
E) 35.5 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
11
Molarity, M, is defined as __________
A) moles of solute dissolved in 1 mol of solvent.
B) moles of solute dissolved in 1 kg of solvent.
C) moles of solute dissolved in 1 L of solvent.
D) moles of solute dissolved in 1 L of solution.
E) moles of solute dissolved in the solution.
A) moles of solute dissolved in 1 mol of solvent.
B) moles of solute dissolved in 1 kg of solvent.
C) moles of solute dissolved in 1 L of solvent.
D) moles of solute dissolved in 1 L of solution.
E) moles of solute dissolved in the solution.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
12
How many grams of solid magnesium chloride, MgCl2, are needed to make 250 mL of 0.50 M solution?
A) 9.5 g
B) 48 g
C) 12 g
D) 125 g
E) 4.8 g
A) 9.5 g
B) 48 g
C) 12 g
D) 125 g
E) 4.8 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
13
Commercial hydrochloric acid is 12.1 M. What volume of commercial HCl solution should be used to prepare 250.0 mL of 3.00 M HCl?
A) 139 mL
B) 126 mL
C) 252 mL
D) 62.0 mL
E) 83.0 mL
A) 139 mL
B) 126 mL
C) 252 mL
D) 62.0 mL
E) 83.0 mL

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
14
A salt solution is added to a marine aquarium. What mass of sodium chloride is needed to produce 250.0 mL of a solution that has a concentration of 0.0500 M ?
A) 731 g
B) 731 mg
C) 58.5 g
D) 2.92 mg
E) 2.92 g
A) 731 g
B) 731 mg
C) 58.5 g
D) 2.92 mg
E) 2.92 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
15
If 100 mL of 3.0 M solution were diluted to 250 mL, what would the concentration be?
A) 0.012 M
B) 0.12 M
C) 1.2 M
D) 12 M
E) 120 M
A) 0.012 M
B) 0.12 M
C) 1.2 M
D) 12 M
E) 120 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
16
If 120 g of NaOH were used to prepare 500 mL of solution, what would the concentration be?
A) 1 M
B) 2 M
C) 3 M
D) 4 M
E) 6 M
A) 1 M
B) 2 M
C) 3 M
D) 4 M
E) 6 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
17
Concentrated sulfuric acid contains 4 g of water for every 100 g of solution. The solvent is __________
A) water.
B) sulfuric acid.
C) concentrated.
D) the same as the solution.
E) the same as the solute in this case.
A) water.
B) sulfuric acid.
C) concentrated.
D) the same as the solution.
E) the same as the solute in this case.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
18
A concentrated aqueous ammonia solution has a density of 0.90 g/mL and is 28.0% by mass ammonia. Determine the molar concentration of this solution.
A) 15 M
B) 1.5 M
C) 0.032 M
D) 31 M
E) 3.0 M
A) 15 M
B) 1.5 M
C) 0.032 M
D) 31 M
E) 3.0 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
19
What volume of 3.0 M NaOH contains 0.15 mole of NaOH?
A) 500 mL
B) 50 mL
C) 5.0 mL
D) 0.50 mL
E) 0.050 mL
A) 500 mL
B) 50 mL
C) 5.0 mL
D) 0.50 mL
E) 0.050 mL

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
20
A homogeneous mixture of two or more substances is called __________
A) a compound.
B) an electrolyte.
C) a solution.
D) a solvent.
E) a mess.
A) a compound.
B) an electrolyte.
C) a solution.
D) a solvent.
E) a mess.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
21
In a demonstration of strong electrolytes, weak electrolytes, and nonelectrolytes, Professor Popsnorkle used a lightbulb apparatus that showed how much a solution conducted electricity by the brightness of the lightbulb. When pure water was tested, the bulb did not light. Then Professor Popsnorkle tested the following aqueous solutions. Which one caused the bulb to burn dimly but not brightly?
A) table salt, NaCl
B) ethanol, CH3CH2OH
C) table sugar, C12H22O11
D) acetic acid, CH3COOH
E) methanol, CH3OH
A) table salt, NaCl
B) ethanol, CH3CH2OH
C) table sugar, C12H22O11
D) acetic acid, CH3COOH
E) methanol, CH3OH

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
22
Hydroxyapatite, [Ca5(PO4)3(OH)], the major component of tooth enamel, is attacked and decomposed by acids more readily than fluorapatite, [Ca5(PO4)3F]. Which one of the following reactions is analogous to the reaction of hydroxyapatite?
A) Ca(s) + 2HCl(aq) CaCl2(s) + 2H+
B) CaF2(s) + 2H+(aq) Ca2+(aq) + 2HF(aq)
C) Ca(OH)2(s) + 2H+(aq) Ca2+(aq) + 2H2O(![<strong>Hydroxyapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH)], the major component of tooth enamel, is attacked and decomposed by acids more readily than fluorapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F]. Which one of the following reactions is analogous to the reaction of hydroxyapatite?</strong> A) Ca(s) + 2HCl(aq) <font face=symbol></font><font face=symbol></font>CaCl<sub>2</sub>(s) + 2H<sup>+</sup> B) CaF<sub>2</sub>(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + 2HF(aq) C) Ca(OH)<sub>2</sub>(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + 2H<sub>2</sub>O( ) D) Ca(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + H<sub>2</sub>(g) E) Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s) + 6H<sup>+</sup>(aq) <font face=symbol></font> 3Ca<sup>2+</sup>(aq) + 2H<sub>3</sub>PO<sub>4</sub>(aq)](https://storage.examlex.com/TB3833/11eaae02_08d5_8580_95d8_7951a72661b8_TB3833_11.jpg) )
)
D) Ca(s) + 2H+(aq) Ca2+(aq) + H2(g)
E) Ca3(PO4)2(s) + 6H+(aq) 3Ca2+(aq) + 2H3PO4(aq)
A) Ca(s) + 2HCl(aq) CaCl2(s) + 2H+
B) CaF2(s) + 2H+(aq) Ca2+(aq) + 2HF(aq)
C) Ca(OH)2(s) + 2H+(aq) Ca2+(aq) + 2H2O(
![<strong>Hydroxyapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH)], the major component of tooth enamel, is attacked and decomposed by acids more readily than fluorapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>F]. Which one of the following reactions is analogous to the reaction of hydroxyapatite?</strong> A) Ca(s) + 2HCl(aq) <font face=symbol></font><font face=symbol></font>CaCl<sub>2</sub>(s) + 2H<sup>+</sup> B) CaF<sub>2</sub>(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + 2HF(aq) C) Ca(OH)<sub>2</sub>(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + 2H<sub>2</sub>O( ) D) Ca(s) + 2H<sup>+</sup>(aq) <font face=symbol></font><font face=symbol></font>Ca<sup>2+</sup>(aq) + H<sub>2</sub>(g) E) Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s) + 6H<sup>+</sup>(aq) <font face=symbol></font> 3Ca<sup>2+</sup>(aq) + 2H<sub>3</sub>PO<sub>4</sub>(aq)](https://storage.examlex.com/TB3833/11eaae02_08d5_8580_95d8_7951a72661b8_TB3833_11.jpg) )
)D) Ca(s) + 2H+(aq) Ca2+(aq) + H2(g)
E) Ca3(PO4)2(s) + 6H+(aq) 3Ca2+(aq) + 2H3PO4(aq)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
23
Which picture best represents an atomic-level view of acetic acid, which is a weak acid, in aqueous solution (water molecules not shown)?
A)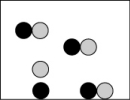
B)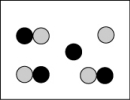
C)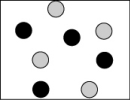
D)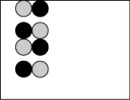
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
24
What is the molar concentration of sodium in a 200.0 mL solution prepared from 1.223 g of sodium phosphate (Na3PO4, 163.9 g/mol), which is a cleaning agent, food additive, and stain remover?
A) 0.03731 M
B) 0.2486 M
C) 0.7338 M
D) 0.1119 M
E) 0.1243 M
A) 0.03731 M
B) 0.2486 M
C) 0.7338 M
D) 0.1119 M
E) 0.1243 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the acid in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 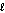 )
)
A) PbCO3(s)
B) CO2(g)
C) PbSO4(s)
D) H2O(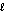 )
)
E) H2SO4(aq)
 )
)A) PbCO3(s)
B) CO2(g)
C) PbSO4(s)
D) H2O(
 )
)E) H2SO4(aq)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the base in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O(  )
)
A) CO32-
B) CO2
C) SO42-
D) H2O
E) H2SO4
 )
)A) CO32-
B) CO2
C) SO42-
D) H2O
E) H2SO4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
27
Chalk contains calcium carbonate. What would be the best solution for cleaning a sidewalk that a preschool class covered with smiling suns, flowers, birds, rainbows, and houses using sidewalk chalk?
A) ammonia
B) plain water
C) vinegar
D) paint thinner
E) olive oil
A) ammonia
B) plain water
C) vinegar
D) paint thinner
E) olive oil

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following statements regarding a weak acid is not correct?
A) A weak acid ionizes only to a small extent in water.
B) A weak acid ionizes in water to produce hydronium ions.
C) A weak acid neutralizes bases.
D) Acetic acid is an example.
E) A weak acid has a very low concentration.
A) A weak acid ionizes only to a small extent in water.
B) A weak acid ionizes in water to produce hydronium ions.
C) A weak acid neutralizes bases.
D) Acetic acid is an example.
E) A weak acid has a very low concentration.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
29
In a demonstration of strong electrolytes, weak electrolytes, and nonelectrolytes, Professor Popsnorkle used a lightbulb apparatus that showed how much a solution conducted electricity by the brightness of the lightbulb. When pure water was tested, the bulb did not light. When some acetic acid was added to the water, the bulb burned dimly. When more acetic acid was added to the solution, the bulb burned a little more brightly. In his frustration to make the bulb shine brightly with acetic acid, Professor Popsnorkle started over by testing the beaker of the pure acetic acid. What was the result?
A) The bulb did not light.
B) The bulb burned dimly.
C) The bulb burned more than any of the others but still not brightly.
D) The bulb burned brightly.
E) Professor Popsnorkle was electrocuted.
A) The bulb did not light.
B) The bulb burned dimly.
C) The bulb burned more than any of the others but still not brightly.
D) The bulb burned brightly.
E) Professor Popsnorkle was electrocuted.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
30
In a demonstration of strong electrolytes, weak electrolytes, and nonelectrolytes, Professor Popsnorkle used a lightbulb apparatus that showed how much a solution conducted electricity by the brightness of the lightbulb. When pure water was tested, the bulb did not light. Then Professor Popsnorkle tested the following aqueous solutions. Which one caused the bulb to burn the brightest?
A) table salt, NaCl
B) ethanol, CH3CH2OH
C) table sugar, C12H22O11
D) acetic acid, CH3COOH
E) methanol, CH3OH
A) table salt, NaCl
B) ethanol, CH3CH2OH
C) table sugar, C12H22O11
D) acetic acid, CH3COOH
E) methanol, CH3OH

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
31
Which contains more solute particles: a 0.10 M aqueous solution of methanol (CH3OH) or a 0.10 M aqueous solution of salt (NaCl)?
A) They contain the same number of solute particles.
B) The salt solution contains twice as many particles as the methanol solution.
C) The methanol solution contains three times as many particles as the salt solution.
D) Neither solution contains solute particles.
E) The methanol solution contains twice as many particles as the salt solution.
A) They contain the same number of solute particles.
B) The salt solution contains twice as many particles as the methanol solution.
C) The methanol solution contains three times as many particles as the salt solution.
D) Neither solution contains solute particles.
E) The methanol solution contains twice as many particles as the salt solution.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
32
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4+. If 5.00 mL of a solution of an ammonia cleaner is titrated directly with 42.6 mL of 0.5000 M HCl, what is the concentration of the NH3 in solution? (Assume that the ammonia is the only solute that reacts with the acid.)
A) 0.0587 M
B) 0.107 M
C) 4.26 M
D) 1.07 M
E) 5.87 M
A) 0.0587 M
B) 0.107 M
C) 4.26 M
D) 1.07 M
E) 5.87 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
33
If the molar concentration of sodium sulfate (Na2SO4) is 0.10 M, what is the concentration of sodium ion?
A) 0.10 M
B) 0.050 M
C) 0.20 M
D) 0.30 M
E) 0.40 M
A) 0.10 M
B) 0.050 M
C) 0.20 M
D) 0.30 M
E) 0.40 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
34
What would be the products in the ionic equation for the following reaction? 2HBr(aq) + 2KOH(aq) products
I. K+(aq) and Br-(aq);
II. KH(aq);
III. H2O(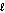 );
);
IV. KBr(s); V. BrOH(aq); VI. KBr(aq)
A) I and III
B) III and IV
C) III and VI
D) II, III, and V
E) II and V
I. K+(aq) and Br-(aq);
II. KH(aq);
III. H2O(
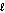 );
); IV. KBr(s); V. BrOH(aq); VI. KBr(aq)
A) I and III
B) III and IV
C) III and VI
D) II, III, and V
E) II and V

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
35
In its reaction with water, ammonia (NH3) __________
A) acts as an acid.
B) acts as a base.
C) acts neither as an acid nor as a base.
D) serves as both an acid and as a base.
E) causes a precipitate to form.
A) acts as an acid.
B) acts as a base.
C) acts neither as an acid nor as a base.
D) serves as both an acid and as a base.
E) causes a precipitate to form.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
36
Which one of the following statements regarding a strong acid is not correct?
A) A strong acid ionizes completely in water.
B) A strong acid ionizes in water to produce hydronium ions.
C) A strong acid neutralizes bases.
D) HCl is an example.
E) A strong acid is highly concentrated.
A) A strong acid ionizes completely in water.
B) A strong acid ionizes in water to produce hydronium ions.
C) A strong acid neutralizes bases.
D) HCl is an example.
E) A strong acid is highly concentrated.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
37
Calcium hydroxide is slightly soluble in water. About 1 gram will dissolve in 1 liter. What are the spectator ions in the reaction of such a dilute solution of calcium hydroxide with hydrochloric acid?
A) Ca2+ and Cl-
B) Ca2+ and OH-
C) H3O+ and OH-
D) H3O+ and Cl-
E) Ca2+, Cl- , H3O+, and OH-
A) Ca2+ and Cl-
B) Ca2+ and OH-
C) H3O+ and OH-
D) H3O+ and Cl-
E) Ca2+, Cl- , H3O+, and OH-

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
38
Two phosphoric acid molecules can combine to make pyrophosphoric acid and another molecule as shown here. The pyrophosphoric acid molecule is a phosphoester and is a structural element in the ADP/ATP metabolic energy system. What molecule is represented by the question mark in the structural reaction equation below? 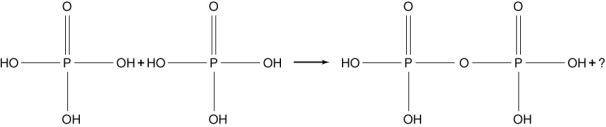
A) O2
B) OH-
C) H2O
D) PO
E) H2

A) O2
B) OH-
C) H2O
D) PO
E) H2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
39
If 50.0 mL of a 0.10 M solution of sodium chloride is mixed with 50.0 mL of 0.10 M magnesium chloride, what is the molar concentration of chloride in the resulting solution?
A) 0.10 M
B) 0.20 M
C) 0.05 M
D) 0.15 M
E) 0.25 M
A) 0.10 M
B) 0.20 M
C) 0.05 M
D) 0.15 M
E) 0.25 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
40
A cross section of a Mad-Dawg gumball shows an outer layer of citric acid and malonic acid, beneath that a layer of food coloring, sugar, and flavoring, beneath that a layer of sodium bicarbonate (NaHCO3), and finally in the middle-gum! A 12-year-old puts one in his mouth and immediately puckers, smiles, and then foams at the mouth before chewing. Where did the foam come from?
A) Twelve-year-olds naturally foam at the mouth.
B) The sodium bicarbonate explodes when exposed to moisture.
C) The acids react with the sodium bicarbonate, making unstable carbonic acid.
D) The foam must have been packed in the gum somewhere.
E) The acids react with the sugar making carbon volcano.
A) Twelve-year-olds naturally foam at the mouth.
B) The sodium bicarbonate explodes when exposed to moisture.
C) The acids react with the sodium bicarbonate, making unstable carbonic acid.
D) The foam must have been packed in the gum somewhere.
E) The acids react with the sugar making carbon volcano.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
41
Sodium metal easily loses an electron to form the sodium ion. In this process, sodium is __________
A) reduced.
B) oxidized.
C) ionized.
D) complexed.
E) both ionized and oxidized.
A) reduced.
B) oxidized.
C) ionized.
D) complexed.
E) both ionized and oxidized.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
42
In a titration, the solution of known concentration delivered by the buret is called the titrant, and the solution being titrated is called the analyte. To carry out a calculation to determine an unknown concentration of a sample from titration data, one would need all of the following data except __________
A) the volume of the titrant delivered.
B) the volume of the analyte.
C) the stoichiometry of the reaction between the titrant and the analyte.
D) the concentration of the analyte.
E) the concentration of the titrant.
A) the volume of the titrant delivered.
B) the volume of the analyte.
C) the stoichiometry of the reaction between the titrant and the analyte.
D) the concentration of the analyte.
E) the concentration of the titrant.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
43
If 1.0 L of 1.0 M HCl spilled and needed to be neutralized, how many grams of the solid sodium carbonate (Na2CO3, 106 g/mol) would be required?
A) 53 g
B) 106 g
C) 1060 g
D) 530 g
E) 212 g
A) 53 g
B) 106 g
C) 1060 g
D) 530 g
E) 212 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
44
What mass of silver chloride will be recovered if excess sodium chloride is added to 500 mL of solution containing 10.79 g of Ag+?
A) 10.8 g
B) 21.6 g
C) 28.6 g
D) 7.2 g
E) 14.3 g
A) 10.8 g
B) 21.6 g
C) 28.6 g
D) 7.2 g
E) 14.3 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
45
Ion-exchange resins can remove Mg2+ and Ca2+ in water (called softening) by releasing Na+. What would be the concentration of sodium ion in the softened water if the combined concentration of these Group 2A ions that were removed from the hard water was 0.026 M?
A) 0.052 M
B) 0.26 M
C) 0.13 M
D) 0.00 M
E) Can't tell, necessary information is lacking.
A) 0.052 M
B) 0.26 M
C) 0.13 M
D) 0.00 M
E) Can't tell, necessary information is lacking.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
46
Sodium thiosulfate (Na2S2O3, molar mass = 158.2 g/mol) is used in photography. Your summer job is at a photography lab and you need to check the purity of an outdated supply. You react 40.21 mL of 0.246 M iodine solution with a 3.232 g sample. What is the percent purity of the sodium thiosulfate that you report to your boss? I2(aq) + 2S2O32-(aq) 2I-(aq) + S4O62-(aq)
A) 100%
B) 48.4%
C) 96.8%
D) 98.6%
E) 84.4%
A) 100%
B) 48.4%
C) 96.8%
D) 98.6%
E) 84.4%

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
47
If one regular antacid tablet contains 500 mg of solid CaCO3 (100 g/mol), how many mL of 1.0 M stomach acid (HCl) could it neutralize?
A) 5 mL
B) 10 mL
C) 50 mL
D) 100 mL
E) 15 mL
A) 5 mL
B) 10 mL
C) 50 mL
D) 100 mL
E) 15 mL

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
48
A reaction that takes place between dissolved barium nitrate and dissolved sodium sulfate results in formation of solid barium sulfate. Which equation describes this reaction?
A) Ba(NO3)2(aq) + Na2SO4(aq) BaSO4(s) + 2NaNO3(aq)
B) BaNO3(aq) + NaSO4(aq) BaSO4(s) + NaNO3(aq)
C) 2Ba(NO3)(aq) + Na2SO4(aq) Ba2SO4(s) + 2NaNO3(aq)
D) Ba(NO3)2(aq) + 2NaSO4(aq) Ba(SO4)2(s) + 2NaNO3(aq)
E) Ba(NO3)2(aq) + Na2SO4(aq) BaSO4(aq) + 2NaNO3(s)
A) Ba(NO3)2(aq) + Na2SO4(aq) BaSO4(s) + 2NaNO3(aq)
B) BaNO3(aq) + NaSO4(aq) BaSO4(s) + NaNO3(aq)
C) 2Ba(NO3)(aq) + Na2SO4(aq) Ba2SO4(s) + 2NaNO3(aq)
D) Ba(NO3)2(aq) + 2NaSO4(aq) Ba(SO4)2(s) + 2NaNO3(aq)
E) Ba(NO3)2(aq) + Na2SO4(aq) BaSO4(aq) + 2NaNO3(s)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
49
In carrying out a titration of a hydrochloric acid solution with a standard sodium hydroxide solution, a student went beyond the end point before reading the volume on the buret. That is, the volume used was larger than the volume required to reach the end point. How will this error affect the calculated concentration of the hydrochloric acid?
A) The calculated concentration will be larger than the actual concentration.
B) The calculated concentration will be smaller than the actual concentration.
C) The calculated concentration will be the correct concentration.
D) There is no way to tell how this error will affect the calculation.
E) The calculated concentration will be the actual concentration.
A) The calculated concentration will be larger than the actual concentration.
B) The calculated concentration will be smaller than the actual concentration.
C) The calculated concentration will be the correct concentration.
D) There is no way to tell how this error will affect the calculation.
E) The calculated concentration will be the actual concentration.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
50
Most chloride salts are soluble. Identify an exception to this generalization.
A) AgCl
B) CaCl2
C) MgCl2
D) BaCl2
E) NaCl
A) AgCl
B) CaCl2
C) MgCl2
D) BaCl2
E) NaCl

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
51
Water-soluble toxic chromium compounds are waste products of electroplating operations, but the chromium can be precipitated as Cr(OH)3 to remediate the water. How much 1.0 M NaOH solution is needed to remove the chromium from 100 L of a solution that is 0.001 M in Cr3+?
A) 100 mL
B) 300 mL
C) 10 L
D) 30 L
E) 33 L
A) 100 mL
B) 300 mL
C) 10 L
D) 30 L
E) 33 L

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
52
Tube worms that survive near geothermal vents of hydrogen sulfide rely on bacteria living inside them to obtain energy by the oxidation of H2S to SO42-. What is the overall change in the oxidation number of sulfur for this reaction?
A) -2
B) +2
C) -6
D) +8
E) +10
A) -2
B) +2
C) -6
D) +8
E) +10

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
53
A 500 mg dietary supplement of L-lysine (an amino acid, 146.2 g/mol) required 68.4 mL of 0.100 M NaOH to reach the end point. How many protons were removed for each L-lysine molecule in this titration?
A) 1
B) 2
C) 3
D) 0.500
E) 2.5
A) 1
B) 2
C) 3
D) 0.500
E) 2.5

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
54
Magnesium hydroxide is insoluble. Write the net ionic equation for the reaction that takes place between a solution of magnesium chloride and a solution of sodium hydroxide.
A) MgCl2(aq) + NaOH(aq) MgOH(s) + NaCl2(aq)
B) MgCl2(aq) + 2NaOH(aq) Mg(OH)2(s) + 2NaCl(aq)
C) Mg2+(aq) + 2OH-(aq) Mg(OH)2(s)
D) Mg2+(aq) + 2Cl- (aq) + 2Na+(aq) + 2OH-(aq) Mg(OH)2(s) + 2NaCl(s)
E) Mg2+(aq) + OH-(aq) MgOH(s)
A) MgCl2(aq) + NaOH(aq) MgOH(s) + NaCl2(aq)
B) MgCl2(aq) + 2NaOH(aq) Mg(OH)2(s) + 2NaCl(aq)
C) Mg2+(aq) + 2OH-(aq) Mg(OH)2(s)
D) Mg2+(aq) + 2Cl- (aq) + 2Na+(aq) + 2OH-(aq) Mg(OH)2(s) + 2NaCl(s)
E) Mg2+(aq) + OH-(aq) MgOH(s)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
55
Hard water contains Mg2+ and Ca2+ ions and tends to form a ring in a bathtub due to its reaction with the soluble anions in soap. The formation of this insoluble material is an example of __________
A) an acid-base reaction.
B) a precipitation reaction.
C) a redox reaction.
D) a bathochromic shift.
E) bad housekeeping.
A) an acid-base reaction.
B) a precipitation reaction.
C) a redox reaction.
D) a bathochromic shift.
E) bad housekeeping.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
56
How many liters of 0.200 M NaOH solution are required to completely react with 1.00 L of 0.100 M HCN solution to produce sodium cyanide and water?
A) 0.25 L
B) 0.50 L
C) 1.00 L
D) 1.50 L
E) 2.00 L
A) 0.25 L
B) 0.50 L
C) 1.00 L
D) 1.50 L
E) 2.00 L

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
57
Silver salts are used in black-and-white photography. Owing to the value of silver, the ions left in developing solutions are often collected by precipitation reactions for recycling. A 4.5 L jug of a developing solution was treated with an excess of sodium chloride, and a precipitate containing 2.86 g of dry silver chloride was collected. What was the molar concentration of the silver ions in the developing solution? The net ionic equation is Ag+(aq) + Cl-(aq) AgCl(s)
A) 2.9 M
B) 5.9 10-3 M
C) 2.9 10-3 M
D) 4.4 10-3 M
E) 4.4 M
A) 2.9 M
B) 5.9 10-3 M
C) 2.9 10-3 M
D) 4.4 10-3 M
E) 4.4 M

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following ionic compounds is insoluble in water?
A) BaCl2
B) BaSO4
C) NaOH
D) Ba(NO3)2
E) MgCl2
A) BaCl2
B) BaSO4
C) NaOH
D) Ba(NO3)2
E) MgCl2

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following compounds is most soluble in water?
A) AgCl
B) CaCO3
C) Al(OH)3
D) K2SO4
E) MgS
A) AgCl
B) CaCO3
C) Al(OH)3
D) K2SO4
E) MgS

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
60
Polymers with ionic functional groups have been developed for removal of ions from water. One example is Amberlite. One form of Amberlite has the structure shown below, where the -SO3H groups act like a weak acid. What type of ions will this polymer attract? 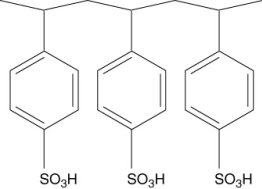
A) cations
B) anions
C) all ions
D) impossible to tell
E) none, since the polymer is neutral

A) cations
B) anions
C) all ions
D) impossible to tell
E) none, since the polymer is neutral

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
61
Potassium is a very reactive metal. Many of its compounds are not very reactive. For example, some class B fire extinguishers contain potassium bicarbonate as a dry powder for use in extinguishing burning liquids. Why is there such a difference in the reactivity of potassium in the bicarbonate salt and potassium that is a combustible metal?
A) The difference in reactivity really is not that great to warrant an explanation.
B) The potassium atom is bonded to an oxygen atom in the bicarbonate salt but is unbonded in the metal and free to react.
C) The potassium metal possesses one additional electron that is easily transferred to other substances; the potassium in the bicarbonate salt already has lost this reactive electron.
D) The potassium in the bicarbonate salt is a base, but the potassium metal is an acid.
E) The potassium in the bicarbonate salt is an acid, but the potassium metal is a base.
A) The difference in reactivity really is not that great to warrant an explanation.
B) The potassium atom is bonded to an oxygen atom in the bicarbonate salt but is unbonded in the metal and free to react.
C) The potassium metal possesses one additional electron that is easily transferred to other substances; the potassium in the bicarbonate salt already has lost this reactive electron.
D) The potassium in the bicarbonate salt is a base, but the potassium metal is an acid.
E) The potassium in the bicarbonate salt is an acid, but the potassium metal is a base.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
62
What is the oxidation number of P in H2PO4-?
A) -5
B) -6
C) 5
D) 6
E) 4
A) -5
B) -6
C) 5
D) 6
E) 4

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
63
In which one of the following compounds is the oxidation number of S equal to +4?
A) H2S
B) S2Cl2
C) MgSO4
D) S8
E) Na2SO3
A) H2S
B) S2Cl2
C) MgSO4
D) S8
E) Na2SO3

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
64
What is meant by the terms solution, solvent, and solute?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
65
The thermite reaction is used in welding applications and chemistry demonstrations because it releases a tremendous amount of energy. Which of the following statements is not correct regarding this reaction? 8Al + 3Fe3O4 9Fe + 4Al2O3
A) Aluminum is oxidized.
B) Iron is reduced.
C) The oxidation number of Al changes from 0 to +3.
D) Aluminum is the reducing agent.
E) Three electrons are transferred from each aluminum atom to each iron atom.
A) Aluminum is oxidized.
B) Iron is reduced.
C) The oxidation number of Al changes from 0 to +3.
D) Aluminum is the reducing agent.
E) Three electrons are transferred from each aluminum atom to each iron atom.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
66
In a spontaneous oxidation-reduction reaction between aluminum and silver ion, the aluminum(III) ion and solid silver are formed. If 0.1 mol of aluminum is consumed in this reaction, how much silver will be produced?
A) 0.3 mol
B) 0.1 mol
C) 1.0 mol
D) 3 mol
E) 0.2 mol
A) 0.3 mol
B) 0.1 mol
C) 1.0 mol
D) 3 mol
E) 0.2 mol

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
67
Magnesium metal burns brightly in the air to make a white powder. What are the likely products?
A) Mg(H2O) and MgO2
B) MgCO3 and MgCO
C) Mg2N3 and Mg2O
D) Mg3N2 and MgO
E) MgNO
A) Mg(H2O) and MgO2
B) MgCO3 and MgCO
C) Mg2N3 and Mg2O
D) Mg3N2 and MgO
E) MgNO

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
68
Chalk is mostly calcium carbonate. Could chalk-dust catch fire and burn in air?
A) Yes, the carbon in the chalk would burn.
B) Yes, calcium is a reactive metal that will burn.
C) No, calcium carbonate is completely oxidized.
D) No, the manufacturer puts a protective coating on chalk so that it will not burn.
E) No, the ignition temperature is extremely high.
A) Yes, the carbon in the chalk would burn.
B) Yes, calcium is a reactive metal that will burn.
C) No, calcium carbonate is completely oxidized.
D) No, the manufacturer puts a protective coating on chalk so that it will not burn.
E) No, the ignition temperature is extremely high.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
69
In which compound does chlorine have an oxidation number of +5?
A) HClO4
B) HClO3
C) HClO2
D) HClO
E) HCl
A) HClO4
B) HClO3
C) HClO2
D) HClO
E) HCl

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
70
The carbon cycle characterizes the cyclic transformations of the element carbon through the environment and biomass. Some of the important species in this cycle are CO2, CO32-, C6H12O6, and CH4. Which of these compounds would undergo complete oxidation by O2 to give the largest change in oxidation number?
A) CO2
B) CO32-
C) C6H12O6
D) CH4
E) CO2 and CO32- are equivalent in this regard.
A) CO2
B) CO32-
C) C6H12O6
D) CH4
E) CO2 and CO32- are equivalent in this regard.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
71
When the oxidation-reduction reaction shown here is balanced, how many electrons are transferred for each atom of copper that reacts? Ag+(aq) + Cu(s) Ag(s) + Cu2+(aq)
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
72
The drinking water standard of the World Health Organization (WHO) for arsenic is 10.0 g/L. What is this standard in parts per billion (ppb)?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
73
Chlorine, ozone, and chlorine dioxide are all common disinfectants for municipal water purification. What chemical property do they all share?
A) They are all strong acids.
B) They are all good oxidizing agents.
C) They are all good reducing agents.
D) They all form precipitates with a wide range of contaminants.
E) They are all strong bases.
A) They are all strong acids.
B) They are all good oxidizing agents.
C) They are all good reducing agents.
D) They all form precipitates with a wide range of contaminants.
E) They are all strong bases.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
74
One type of alcohol breathalyzers used by law enforcement involves the following reaction. The amount of ethanol present is determined by a change in color of the solution. What type of reaction is this? 3CH3CH2OH(g) + 2K2Cr2O7(aq) + 8H2SO4(aq)
3CH3COOH(aq) + 2Cr2(SO4)3(aq) + 2K2SO4(aq) + 11H2O
A) base ionization
B) acid ionization
C) acid-base neutralization
D) oxidation-reduction
E) dissociation
3CH3COOH(aq) + 2Cr2(SO4)3(aq) + 2K2SO4(aq) + 11H2O
A) base ionization
B) acid ionization
C) acid-base neutralization
D) oxidation-reduction
E) dissociation

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
75
The half-reaction for the reduction of molecular oxygen to water is __________
A)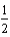 O2(g) H2O(
O2(g) H2O(  ).
).
B)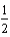 O2(g) + H2(g) H2O(
O2(g) + H2(g) H2O(  ).
).
C)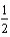 O2(g) + H2(g) + 2e- H2O(
O2(g) + H2(g) + 2e- H2O(  ).
).
D)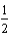 O2(g) + 2 H+(g) + 2e- H2O(
O2(g) + 2 H+(g) + 2e- H2O(  ).
).
E)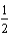 O2(g) + 2 H+(g) H2O(
O2(g) + 2 H+(g) H2O( 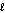 ) + 2e-.
) + 2e-.
A)
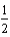 O2(g) H2O(
O2(g) H2O( 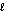 ).
).B)
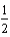 O2(g) + H2(g) H2O(
O2(g) + H2(g) H2O(  ).
).C)
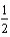 O2(g) + H2(g) + 2e- H2O(
O2(g) + H2(g) + 2e- H2O(  ).
).D)
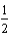 O2(g) + 2 H+(g) + 2e- H2O(
O2(g) + 2 H+(g) + 2e- H2O(  ).
).E)
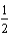 O2(g) + 2 H+(g) H2O(
O2(g) + 2 H+(g) H2O(  ) + 2e-.
) + 2e-.
Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
76
Lead sinkers used for saltwater fishing do not react with sodium ions in the water. From this we can infer that __________
A) lead can reduce sodium ions.
B) sodium ions can reduce lead.
C) lead is heavier than sodium.
D) sodium ions cannot oxidize lead.
E) sodium ions cannot reduce lead.
A) lead can reduce sodium ions.
B) sodium ions can reduce lead.
C) lead is heavier than sodium.
D) sodium ions cannot oxidize lead.
E) sodium ions cannot reduce lead.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
77
The oxidation number of carbon in carbon dioxide is
A) +2
B) -2
C) +4
D) -4
E) +1
A) +2
B) -2
C) +4
D) -4
E) +1

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
78
Nitrogen-fixing bacteria and plants are capable of converting atmospheric nitrogen to ammonia. This process is an example of __________
A) an acid-base reaction.
B) a precipitation reaction.
C) an oxidation reaction.
D) a reduction reaction.
E) ion exchange.
A) an acid-base reaction.
B) a precipitation reaction.
C) an oxidation reaction.
D) a reduction reaction.
E) ion exchange.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
79
Methane (CH4) is a suitable fuel for burning because it is readily oxidized by oxygen gas, forming carbon dioxide and water. Hydrogen is also a good fuel for burning with oxygen gas, forming water as a product. What change in oxidation number always accompanies oxidation?
A) a decrease
B) an increase
C) no change
D) The change varies with the reaction.
E) Oxidation number increases by 1 unit.
A) a decrease
B) an increase
C) no change
D) The change varies with the reaction.
E) Oxidation number increases by 1 unit.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
80
In the following reactions, which element is oxidized? Cu + FeSO4 Fe + CuSO4
A) Cu
B) Fe
C) S
D) O
E) None, this is not a redox reaction.
A) Cu
B) Fe
C) S
D) O
E) None, this is not a redox reaction.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck


