Exam 4: Solution Chemistry and the Hydrosphere
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
When the oxidation-reduction reaction shown here is balanced, how many electrons are transferred for each atom of copper that reacts? Ag+(aq) + Cu(s) Ag(s) + Cu2+(aq)
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Chalk is mostly calcium carbonate. Could chalk-dust catch fire and burn in air?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
C
Identify the base in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 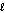 )
)
Free
(Multiple Choice)
4.7/5  (41)
(41)
Correct Answer:
A
Magnesium metal burns brightly in the air to make a white powder. What are the likely products?
(Multiple Choice)
4.7/5  (40)
(40)
What volume of 12 M HCl solution needs to be diluted to produce 500 mL of 3.0 M HCl solution?
(Multiple Choice)
5.0/5  (37)
(37)
Commercial hydrochloric acid is 12.1 M. What volume of commercial HCl solution should be used to prepare 250.0 mL of 3.00 M HCl?
(Multiple Choice)
4.8/5  (30)
(30)
The thermite reaction is used in welding applications and chemistry demonstrations because it releases a tremendous amount of energy. Which of the following statements is not correct regarding this reaction? 8Al + 3Fe3O4 9Fe + 4Al2O3
(Multiple Choice)
4.9/5  (42)
(42)
A chemistry student attempted to make a 0.2000 M solution of NaCl using a 100.0 mL volumetric flask. She added exactly 100.0 mL of water to the flask, then added 0.02000 mol of NaCl, and found that the total volume in the flask was above the 100.0 mL mark. What was the concentration of the solution?
(Multiple Choice)
4.8/5  (34)
(34)
Sodium fluoride is added to drinking water in some municipalities to protect teeth against cavities. The target is to convert hydroxyapatite, Ca10(PO4)6(OH)2, into more stable fluorapatite, Ca10(PO4)6F2. The EPA currently restricts the concentration of fluoride in drinking water to 4 mg/L. Express this concentration in parts per million (ppm).
(Short Answer)
4.8/5  (27)
(27)
A salt solution is added to a marine aquarium. What mass of sodium chloride is needed to produce 250.0 mL of a solution that has a concentration of 0.0500 M ?
(Multiple Choice)
4.7/5  (33)
(33)
Nitrogen-fixing bacteria and plants are capable of converting atmospheric nitrogen to ammonia. This process is an example of __________
(Multiple Choice)
4.9/5  (40)
(40)
One type of alcohol breathalyzers used by law enforcement involves the following reaction. The amount of ethanol present is determined by a change in color of the solution. What type of reaction is this? 3CH3CH2OH(g) + 2K2Cr2O7(aq) + 8H2SO4(aq)
3CH3COOH(aq) + 2Cr2(SO4)3(aq) + 2K2SO4(aq) + 11H2O
(Multiple Choice)
4.8/5  (42)
(42)
Which one of the following statements regarding a strong acid is not correct?
(Multiple Choice)
4.8/5  (42)
(42)
Which one of the following statements regarding a weak acid is not correct?
(Multiple Choice)
4.7/5  (35)
(35)
A homogeneous mixture of two or more substances is called __________
(Multiple Choice)
4.9/5  (40)
(40)
In the dilution of 10.0 mL of a 0.10 M solution of HCl to a volume of 20.0 mL, what remains unchanged?
(Multiple Choice)
4.8/5  (34)
(34)
What would be the products in the ionic equation for the following reaction? 2HBr(aq) + 2KOH(aq) products
I. K+(aq) and Br-(aq);
II. KH(aq);
III. H2O( 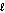 );
IV. KBr(s); V. BrOH(aq); VI. KBr(aq)
);
IV. KBr(s); V. BrOH(aq); VI. KBr(aq)
(Multiple Choice)
4.7/5  (39)
(39)
If the molar concentration of sodium sulfate (Na2SO4) is 0.10 M, what is the concentration of sodium ion?
(Multiple Choice)
5.0/5  (38)
(38)
In a titration, the solution of known concentration delivered by the buret is called the titrant, and the solution being titrated is called the analyte. To carry out a calculation to determine an unknown concentration of a sample from titration data, one would need all of the following data except __________
(Multiple Choice)
4.8/5  (44)
(44)
Showing 1 - 20 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)