Deck 17: Additional Aspects of Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 17: Additional Aspects of Acid-Base Equilibria
1
In 0.100 M HC2H3O2(aq), [H3O+(aq)] = [C2H3O2-(aq)] = 1.3 x 10-3 M. If a few drops of concentrated HCl(aq) are added to this solution, the C2H3O2-(aq) concentration is:
A) < 1.3 x 10-3 M
B) > 1.3 x 10-3 M
C) = 1.3 x 10-3 M
D) 0.100 M
A) < 1.3 x 10-3 M
B) > 1.3 x 10-3 M
C) = 1.3 x 10-3 M
D) 0.100 M
< 1.3 x 10-3 M
2
The pH of a buffer solution changes only slightly with addition of a small amount of acid or base.
True
3
In the titration of a solution of HCN(aq) with NaOH(aq), the equivalence point occurs at a pH greater than 7.
True
4
What is the [H3O+] of a solution measured to be 0.20 M in sodium acetate and 0.40 M in acetic acid? [Ka = 1.8 × 10-5]
A) 1.8 × 10-5 M
B) 9.0 × 10-6M
C) 3.6 × 10-5 M
D) 7.2 × 10-5M
E) 4.7 M
A) 1.8 × 10-5 M
B) 9.0 × 10-6M
C) 3.6 × 10-5 M
D) 7.2 × 10-5M
E) 4.7 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which among the following pairs is inefficient as buffer pair?
A) ammonium chloride and ammonium hydroxide
B) sodium chloride and sodium hydroxide
C) boric acid and sodium borate
D) potassium carbonate and potassium bicarbonate
E) potassium bromide and hydrobromic acid
A) ammonium chloride and ammonium hydroxide
B) sodium chloride and sodium hydroxide
C) boric acid and sodium borate
D) potassium carbonate and potassium bicarbonate
E) potassium bromide and hydrobromic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
A salt of a polyprotic acid such as NaHCO3 cannot act as an acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
The color change range of most acid-base indicators is 1 pH unit.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
The common ion in a mixture of a weak acid and a strong acid is the hydronium ion.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
How will addition of sodium acetate to an acetic acid solution affect the pH?
A) It will lower the pH.
B) The pH will not change.
C) The solution becomes hotter.
D) The pH cannot be measured.
E) It will raise the pH.
A) It will lower the pH.
B) The pH will not change.
C) The solution becomes hotter.
D) The pH cannot be measured.
E) It will raise the pH.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Acid-base indicators have two forms: an acid of one color and a base of another color.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
How will addition of sodium chloride affect the pH of a HCl solution?
A) It will lower the pH.
B) The pH will not change.
C) The solution becomes hotter.
D) The pH cannot be measured.
E) It will raise the pH.
A) It will lower the pH.
B) The pH will not change.
C) The solution becomes hotter.
D) The pH cannot be measured.
E) It will raise the pH.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
For an accurate titration, the end point needs to match the equivalence point.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
The common ion in a mixture of a weak base and a strong base is the hydronium ion.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
A weak acid-strong base will produce a longer vertical section of a titration curve than will a strong acid-strong base.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
What is the concentration of the acetate ion of a solution measured to be 0.20 M acetic acid and 0.20 M in hydrochloric acid? [Ka for acetic acid = 1.8 × 10-5]
A) 3.6 × 10-5 M
B) 9.0 × 10-6 M
C) 1.8 × 10-5 M
D) 7.2 × 10-5 M
E) 0.20 M
A) 3.6 × 10-5 M
B) 9.0 × 10-6 M
C) 1.8 × 10-5 M
D) 7.2 × 10-5 M
E) 0.20 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
A strong acid and its conjugate base will form a buffer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The pH of a buffer depends mainly on the pKa of the weak acid component of the buffer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Ten milliliters of 0.10 M NH3(aq) (K = 1.8 × 10-5) is mixed with 10 mL of 0.10 M NH4Cl. Neglecting the differences between activities and concentrations, the resulting solution:
A) has a pH = 4.74
B) has a [H+] of approximately 10-3 M
C) has a [NH4+] greater than that of the NH4Cl(aq)
D) has an [OH-] of 1.8 × 10-5 M
E) is acidic
A) has a pH = 4.74
B) has a [H+] of approximately 10-3 M
C) has a [NH4+] greater than that of the NH4Cl(aq)
D) has an [OH-] of 1.8 × 10-5 M
E) is acidic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
If some NH4Cl is added to an aqueous solution of NH3:
A) the pH of the solution will increase
B) the pH of the solution will decrease
C) the solution will not have pH
D) the pH of the solution will not change
E) NH4Cl cannot be added to NH3
A) the pH of the solution will increase
B) the pH of the solution will decrease
C) the solution will not have pH
D) the pH of the solution will not change
E) NH4Cl cannot be added to NH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
A solution of sodium carbonate is easier to calculate the pH than sodium hydrogen carbonate because there is only one hydrolysis reaction instead of two.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
Phenolphthalein may be used as an indicator for the titration of:
A) a weak base with a strong acid
B) a weak acid with a weak base
C) any acid and base
D) a weak acid with a strong base
E) phenolphthalein cannot be used as an indicator
A) a weak base with a strong acid
B) a weak acid with a weak base
C) any acid and base
D) a weak acid with a strong base
E) phenolphthalein cannot be used as an indicator

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
What factor governs the selection of an indicator for a neutralization titration?
A) the final volume of the solution
B) the volume of titrant
C) the molarity of the standard solution
D) the pH at the stoichiometric (equivalence) point
E) the solubility of the indicator
A) the final volume of the solution
B) the volume of titrant
C) the molarity of the standard solution
D) the pH at the stoichiometric (equivalence) point
E) the solubility of the indicator

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following mixtures would you dismiss as a potential buffer in a laboratory?
A) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.10 M HCl(aq).
B) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.050 M HCl(aq).
C) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.10 M HC2H3O2(aq).
D) mixing equal volumes of 0.10 M HC2H3O2(aq) and 0.050 M NaOH(aq).
A) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.10 M HCl(aq).
B) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.050 M HCl(aq).
C) mixing equal volumes of 0.10 M NaC2H3O2(aq) and 0.10 M HC2H3O2(aq).
D) mixing equal volumes of 0.10 M HC2H3O2(aq) and 0.050 M NaOH(aq).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
In the titration of 50.0 mL of 0.0200 M C6H5COOH(aq) with 0.100 M NaOH(aq), what is/are the major species in the solution after the addition of 5.0 mL of NaOH(aq)?
A) C6H5COOH, C6H5COO-, and Na+
B) C6H5COOH
C) C6H5COO- and Na+
D) C6H5COOH, OH-, and Na+
A) C6H5COOH, C6H5COO-, and Na+
B) C6H5COOH
C) C6H5COO- and Na+
D) C6H5COOH, OH-, and Na+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.10 M NH4NO3
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following can act as buffer solutions?
I. 0.1 M HC2H3O2/0.1 M NaC2H3O2
II. 0.1 M NH3/0.1 M NH4Cl
III. 0.1 M HNO3/0.1 M NaNO3
IV. 0.1 M H2SO3/0.1 M NaHSO3
V. 0.1 M KHSO4/ 0.1 M H2SO4
A) I), II), and III)
B) II), III) and IV)
C) III) and IV)
D) I), II) and IV)
E) III), IV) and V)
I. 0.1 M HC2H3O2/0.1 M NaC2H3O2
II. 0.1 M NH3/0.1 M NH4Cl
III. 0.1 M HNO3/0.1 M NaNO3
IV. 0.1 M H2SO3/0.1 M NaHSO3
V. 0.1 M KHSO4/ 0.1 M H2SO4
A) I), II), and III)
B) II), III) and IV)
C) III) and IV)
D) I), II) and IV)
E) III), IV) and V)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.1 M NaCl
A) red
B) yellow
C) red-yellow mixture
D) orange
E) The indicator keeps its original colour.
A) red
B) yellow
C) red-yellow mixture
D) orange
E) The indicator keeps its original colour.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
The following compounds are available as 0.10 M aqueous solutions: pyridine (pKb = 8.82), triethylamine (pKb = 3.25), HClO4, NaOH, phenol (pKa = 9.96), HClO (pKa = 7.54), and NH3 (pKb = 4.74). Identify two solutions that could be used to prepare a buffer with a pH of approximately 5.
A) pyridine and HClO4
B) triethyamine and HClO4
C) phenol and NaOH
D) HClO and NaOH
A) pyridine and HClO4
B) triethyamine and HClO4
C) phenol and NaOH
D) HClO and NaOH

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
A solution containing equimolar amounts of a weak acid with Ka = 10-5 and its sodium salt has:
A) pH > 7
B) pH < 7
C) pH = 7
D) pH dependent on concentration ratios
E) pH dependent on the nature of the acid anion
A) pH > 7
B) pH < 7
C) pH = 7
D) pH dependent on concentration ratios
E) pH dependent on the nature of the acid anion

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements correctly describe a typical titration curve for the titration of a strong acid by a strong base?
I. The beginning pH is low.
II. The pH change is slow until near the equivalence point.
III. At the equivalence point, pH changes by a large value.
IV. Beyond the equivalence point, pH rises rapidly.
V. The equivalence point would be at a pH less than 3.5.
A) I), III) and V)
B) II), III) and IV)
C) I), III) and IV)
D) III), IV) and V)
E) I), II) and III)
I. The beginning pH is low.
II. The pH change is slow until near the equivalence point.
III. At the equivalence point, pH changes by a large value.
IV. Beyond the equivalence point, pH rises rapidly.
V. The equivalence point would be at a pH less than 3.5.
A) I), III) and V)
B) II), III) and IV)
C) I), III) and IV)
D) III), IV) and V)
E) I), II) and III)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the correct statement.
A) 30 mL of 2 molar H3PO4 will exactly react with 15 mL of a 2 molar NaOH solution.
B) One liter of 1 molar HCl will exactly neutralize 2 liters of 0.5 molar NaOH.
C) A 1 molal solution always contains exactly 1 mole in a liter of solution.
D) One liter of a 1 molar solution of an acid always exactly neutralizes one liter of a one molar solution of a base.
E) A 1 molar solution requires 2 moles of H2SO4 per liter of solution.
A) 30 mL of 2 molar H3PO4 will exactly react with 15 mL of a 2 molar NaOH solution.
B) One liter of 1 molar HCl will exactly neutralize 2 liters of 0.5 molar NaOH.
C) A 1 molal solution always contains exactly 1 mole in a liter of solution.
D) One liter of a 1 molar solution of an acid always exactly neutralizes one liter of a one molar solution of a base.
E) A 1 molar solution requires 2 moles of H2SO4 per liter of solution.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.10 M NaCN
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
The solution that is added from the burret during a titration is the:
A) buffer
B) titrant
C) indicator
D) base
E) titrator
A) buffer
B) titrant
C) indicator
D) base
E) titrator

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
What is the buffer range (for an effective 2.0 pH unit) for a benzoic acid/sodium benzoate buffer? [Ka for benzoic acid is 6.3 × 10-5]
A) 8.8 - 10.8
B) 7.4 - 9.4
C) 5.3 - 7.3
D) 4.7 - 6.7
E) 3.2 - 5.2
A) 8.8 - 10.8
B) 7.4 - 9.4
C) 5.3 - 7.3
D) 4.7 - 6.7
E) 3.2 - 5.2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The Henderson-Hasselbach equation, used to calculate the pH of simple conjugate-pair buffer systems, would be expressed for an ammonia/ammonium chloride buffer, for which Kb(NH3) is 1.8 × 10-5, as:
A) pH = 4.74 + log([NH3]/[NH4+])
B) pH = 4.74 + log([NH4+]/[NH3])
C) pH = 9.25 + log([NH3]/[NH4+])
D) pH = 9.25 + log([NH4+]/[NH3])
E) pH = 14.0 - log(1.8 × 10-5)
A) pH = 4.74 + log([NH3]/[NH4+])
B) pH = 4.74 + log([NH4+]/[NH3])
C) pH = 9.25 + log([NH3]/[NH4+])
D) pH = 9.25 + log([NH4+]/[NH3])
E) pH = 14.0 - log(1.8 × 10-5)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.20 M KOH
A) red
B) yellow
C) red-yellow mixture
D) orange
E) The indicator keeps its original colour.
A) red
B) yellow
C) red-yellow mixture
D) orange
E) The indicator keeps its original colour.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Phenol red indicator changes from yellow to red in the pH range from 6.6 to 8.0. State what color the indicator will assume in the following solution: 0.10 M HC2H3O2
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.
A) red
B) yellow
C) red-yellow mixture
D) The indicator is its original color.
E) There is not enough information to answer this question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
For the following titration, determine whether the solution at the equivalence point is acidic, basic or neutral and why: HCl is titrated with NH3(aq)
A) acidic because of hydrolysis of NH4+
B) basic because of hydrolysis of NH3
C) acidic because of hydrolysis of Cl-
D) acidic because of hydrolysis of HCl
E) neutral salt of strong acid and strong base
A) acidic because of hydrolysis of NH4+
B) basic because of hydrolysis of NH3
C) acidic because of hydrolysis of Cl-
D) acidic because of hydrolysis of HCl
E) neutral salt of strong acid and strong base

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the pH indicators from the list above would be most appropriate for the titration of 0.30 M acetic acid (Ka = 1.8 × 10-5) with 0.15 M sodium hydroxide?
A) methyl orange
B) litmus
C) thymol blue
D) trinitrobenzene
E) Both thymol blue and litmus can be used.
A) methyl orange
B) litmus
C) thymol blue
D) trinitrobenzene
E) Both thymol blue and litmus can be used.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Which condition characterizes the stoichiometric point of a neutralization titration?
A) Equivalent amounts of acid and base have reacted.
B) The pH is exactly 7.0.
C) The indicator changes color.
D) A slight excess of titrant is present.
E) A slight excess of indicator is present.
A) Equivalent amounts of acid and base have reacted.
B) The pH is exactly 7.0.
C) The indicator changes color.
D) A slight excess of titrant is present.
E) A slight excess of indicator is present.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the pH of a 1.00 L solution of 0.100 M NH3(aq) after the addition of 0.010 mol NH4Cl(s). For NH3, pKb = 4.74.
A) 10.26
B) 9.26
C) 8.26
D) 11.56
A) 10.26
B) 9.26
C) 8.26
D) 11.56

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
A weak acid has Ka = 1.00 × 10-3. If [HA] = 1.00 M what must be [A-] for the pH to be 2.7?
A) 0.50 M
B) 2.0 M
C) 2.7 M
D) 0.37 M
E) 0.75 M
A) 0.50 M
B) 2.0 M
C) 2.7 M
D) 0.37 M
E) 0.75 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Determine the [C2H3O2-] of the following solution. Initial concentrations are given. [HC2H3O2] = 0.250 M, [HI] = 0.120 M [Ka = 1.8 × 10-5]
A) 8.6 × 10-6 M
B) 3.8 × 10-5 M
C) 1.8 × 10-5 M
D) 0.25 M
E) 0.37 M
A) 8.6 × 10-6 M
B) 3.8 × 10-5 M
C) 1.8 × 10-5 M
D) 0.25 M
E) 0.37 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
The titration curve for 10.0 mL of 0.100 M H3PO4(aq) with 0.100 M NaOH(aq) is given below. 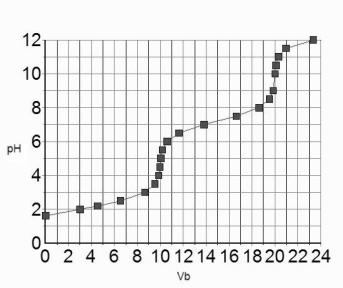 Estimate the pKa2 of H3PO4.
Estimate the pKa2 of H3PO4.
A) 7.2
B) 4.8
C) 9.8
D) 2.2
 Estimate the pKa2 of H3PO4.
Estimate the pKa2 of H3PO4.A) 7.2
B) 4.8
C) 9.8
D) 2.2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the pH of the following solution. Initial concentrations are given. [HF] = 1.296 M, [NaF] = 1.045 M, Ka for HF is 6.6 × 10-4
A) 10.73
B) 3.27
C) 3.18
D) 3.09
E) 11.91
A) 10.73
B) 3.27
C) 3.18
D) 3.09
E) 11.91

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
In the neutralization of 50.0 mL of 0.1 M BOH (a weak base with Kb = 1.6 × 10-7) with 0.10 M H2SO4, the most correct description of the solution at the mid-point of the titration, i.e., half neutralized, is:
A) a solution in which [B+] essentially equals [BOH]
B) a solution in which [B+] essentially equals 4 × 10-4 M
C) a solution whose volume is 75 mL and contains some undissociated BOH molecules and some B+, OH-, and HSO4- ions
D) a solution containing SO42- and B2+ ions
E) a solution in contact with BSO4 solid
A) a solution in which [B+] essentially equals [BOH]
B) a solution in which [B+] essentially equals 4 × 10-4 M
C) a solution whose volume is 75 mL and contains some undissociated BOH molecules and some B+, OH-, and HSO4- ions
D) a solution containing SO42- and B2+ ions
E) a solution in contact with BSO4 solid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the pH of the following solution. Initial concentrations are given. [HC2H3O2] = 0.250 M, [HCl] = 0.120 M [Ka = 1.8 × 10-5]
A) 0.60
B) 0.92
C) 0.43
D) 4.74
E) 13.08
A) 0.60
B) 0.92
C) 0.43
D) 4.74
E) 13.08

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
For the following titration, determine whether the solution at the equivalence point is acidic, basic, or neutral and why: NaHCO3(aq) titrated with NaOH(aq)
A) basic because of excess OH-
B) acidic because of hydrolysis of HCO3-
C) acidic because of hydrolysis of Na+
D) neutral salt of strong acid and strong base
E) basic because of hydrolysis of CO32-
A) basic because of excess OH-
B) acidic because of hydrolysis of HCO3-
C) acidic because of hydrolysis of Na+
D) neutral salt of strong acid and strong base
E) basic because of hydrolysis of CO32-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
For the following titration, determine whether the solution at the equivalence point is acidic, basic or neutral and why: KOH is titrated with HI(aq)
A) basic because of hydrolysis of K+
B) basic because of hydrolysis of KOH
C) acidic because of hydrolysis of OH-
D) acidic because of hydrolysis of HI
E) neutral salt of strong acid and strong base
A) basic because of hydrolysis of K+
B) basic because of hydrolysis of KOH
C) acidic because of hydrolysis of OH-
D) acidic because of hydrolysis of HI
E) neutral salt of strong acid and strong base

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Why do we avoid titrating ammonia with acetic acid?
A) The change in pH near the end point is not large enough to be accurately detected.
B) There is no known indicator which changes color at the right pH.
C) The reaction is too slow.
D) There is not enough difference between the ionization constants of ammonia and acetic acid.
E) Ammonia does not react with acetic acid.
A) The change in pH near the end point is not large enough to be accurately detected.
B) There is no known indicator which changes color at the right pH.
C) The reaction is too slow.
D) There is not enough difference between the ionization constants of ammonia and acetic acid.
E) Ammonia does not react with acetic acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the pH of the following solution. Initial concentrations are given. [HC2H3O2] = 0.250 M, [NaC2H3O2] = 0.120 M [Ka = 1.8 × 10-5]
A) 5.1
B) 4.4
C) 8.9
D) 9.6
E) 7.0
A) 5.1
B) 4.4
C) 8.9
D) 9.6
E) 7.0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the expression that gives the molar concentration of a H2SO4 solution if 24.3 mL of a 0.105 M NaOH solution is required to titrate 60 mL of the acid.
A) (60 × 24.3)/0.105
B) (60 × 2)/(24.3 × 0.105)
C) (24.3 × 0.105)/(60)
D) (24.3 × 0.105)/(60 × 2)
E) (60 × 0.105)/(2 × 24.3)
A) (60 × 24.3)/0.105
B) (60 × 2)/(24.3 × 0.105)
C) (24.3 × 0.105)/(60)
D) (24.3 × 0.105)/(60 × 2)
E) (60 × 0.105)/(2 × 24.3)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
A solution of an unknown acid had a pH of 3.70. Titration of a 25.0 mL aliquot of the acid solution required 21.7 mL of 0.104 M sodium hydroxide for complete reaction. Assuming that the acid is monoprotic, what is its ionization constant?
A) 9.0 × 10-2
B) 2.0 × 10-4
C) 4.4 × 10-7
D) 3.6 × 10-9
E) 2.7 × 10-11
A) 9.0 × 10-2
B) 2.0 × 10-4
C) 4.4 × 10-7
D) 3.6 × 10-9
E) 2.7 × 10-11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Assuming no volume change on mixing, what mass of ammonium chloride should be added to 250.0 mL of 0.25 M ammonia to produce a solution of pH 10.70? [Kb for ammonia is 1.8 × 10-5]
A) 3.7 mg
B) 120 mg
C) 40 mg
D) 30 mg
E) 80 mg
A) 3.7 mg
B) 120 mg
C) 40 mg
D) 30 mg
E) 80 mg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
What is the pH of a solution prepared by mixing equal volumes of 0.10 M hydrochloric and 0.1 M hydrofluoric acid? [Ka for HF is 6.6 × 10-4]
A) 1.0
B) 1.3
C) 1.6
D) 2.2
E) 5.0
A) 1.0
B) 1.3
C) 1.6
D) 2.2
E) 5.0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the pH of a 1.00 L solution of 0.100 M NH3(aq) after the addition of 0.010 mol HCl(g). For NH3, pKb = 4.74.
A) 10.21
B) 9.26
C) 8.31
D) 11.46
A) 10.21
B) 9.26
C) 8.31
D) 11.46

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Determine the [F-] of the following solution. Initial concentrations are given. [HF] = 1.296 M, [NaF] = 1.045 M, Ka for HF is 6.6 × 10-4
A) 1.046 M
B) 2.344 M
C) 5.3 × 10-4 M
D) 8.2 × 10-4 M
E) 0.251 M
A) 1.046 M
B) 2.344 M
C) 5.3 × 10-4 M
D) 8.2 × 10-4 M
E) 0.251 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
A weak acid has Ka = 4.2 × 10-3. If [A-] = 2.0 M, what must [HA] be so that [H+] = 2.1 × 10-3 M?
A) 1.5 M
B) 2.0 M
C) 1.0 M
D) 0.5 M
E) 0.25 M
A) 1.5 M
B) 2.0 M
C) 1.0 M
D) 0.5 M
E) 0.25 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Determine the pH of the following solution. Initial concentrations are given. [HF] = 1.296 M, [HCl] = 1.045 M, Ka for HF is 6.6 × 10-4
A) 14
B) 3.1
C) 3.2
D) -0.019
E) 0.60
A) 14
B) 3.1
C) 3.2
D) -0.019
E) 0.60

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
In a solution prepared by mixing equal volumes of 0.20 M acetic acid and 0.20 M hydrobromic acid, the common ion is ________.
A) Br-
B) C2H3O22-
C) H2C2H3O2
D) H2Br+
E) H3O+
A) Br-
B) C2H3O22-
C) H2C2H3O2
D) H2Br+
E) H3O+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
What is the change in pH after addition of 10.0 mL of 1.0 M sodium hydroxide to 90.0 mL of a 1.0 M NH3/1.0 M NH4+ buffer? [Kb for ammonia is 1.8 × 10-5]
A) 1.0 pH unit
B) 0.1 pH unit
C) 0.01 pH unit
D) 0.001 pH unit
E) zero
A) 1.0 pH unit
B) 0.1 pH unit
C) 0.01 pH unit
D) 0.001 pH unit
E) zero

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
How many mL of 0.200 M acetic acid are mixed with 13.2 mL of 0.200 M sodium acetate to give a buffer with pH = 4.2?
A) 37 mL
B) 18 mL
C) 3.8 mL
D) 14 mL
E) 46 mL
A) 37 mL
B) 18 mL
C) 3.8 mL
D) 14 mL
E) 46 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
A solution has [HC7H5O2] = 0.100 M and [Ca(C7H5O2)2] = 0.200 M. Ka = 6.3 × 10-5 for HC7H5O2. The solution volume is 5.00 L. What is the pH of the solution after 10.00 ml of 5.00 M NaOH is added?
A) 4.80
B) 4.86
C) 4.65
D) 4.70
E) 4.75
A) 4.80
B) 4.86
C) 4.65
D) 4.70
E) 4.75

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
What is the pH of a solution made by dissolving 2.16 g of sodium benzoate (NaC6H5CO2) in a sufficient volume of 0.033 M benzoic acid solution to prepare 500.0 mL of buffer? [Ka for benzoic acid is 6.3 × 10-5]
A) 4.16
B) 4.37
C) 4.64
D) 5.77
E) 6.30
A) 4.16
B) 4.37
C) 4.64
D) 5.77
E) 6.30

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
What mass of sodium acetate should be dissolved in 250.0 mL of 0.30 M acetic acid to form a buffer of pH 5.0? [Ka for acetic acid is 1.8 × 10-5]
A) 11 g
B) 8.0 g
C) 7.5 g
D) 5.0 g
E) 1.4 g
A) 11 g
B) 8.0 g
C) 7.5 g
D) 5.0 g
E) 1.4 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
A buffer was prepared by adding 2.4 g of ammonium nitrate to 100.0 mL of 0.30 M ammonia (Kb = 1.8 × 10-5). To this solution was then added 10.0 mL of 0.30 M sodium hydroxide, which caused a pH change of ________.
A) 3.0 pH units
B) 0.30 pH units
C) 0.90 pH units
D) 0.09 pH units
E) zero
A) 3.0 pH units
B) 0.30 pH units
C) 0.90 pH units
D) 0.09 pH units
E) zero

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
A solution has [HC7H5O2] = 0.100 M and [Ca(C7H5O2)2] = 0.200 M. Ka = 6.3 × 10-5 for HC7H5O2. The solution volume is 5.00 L. What is the pH of this solution after 5.00 ml 10.0 M HCl is added?
A) 4.80
B) 4.86
C) 4.65
D) 4.70
E) 4.75
A) 4.80
B) 4.86
C) 4.65
D) 4.70
E) 4.75

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
For HClO2, Ka = 1.2 × 10-2. What is the pH of a solution in which [NaClO2] = 0.193 M and [HClO2] = 0.203 M?
A) 3.32
B) 0.11
C) 1.92
D) 1.94
E) 1.90
A) 3.32
B) 0.11
C) 1.92
D) 1.94
E) 1.90

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
A handbook states that to prepare a particular buffer solution mix 39.0 mL of 0.20 M NaH2PO4 with 61.0 mL of 0.20 M Na2HPO4. What will be the pH of this buffer?
A) 7.2
B) 7.4
C) 7.0
D) 6.8
E) 6.6
A) 7.2
B) 7.4
C) 7.0
D) 6.8
E) 6.6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Twenty-five milliliters of 0.10 M HCl is titrated with 0.10 M NaOH. What is the pH at equivalence?
A) 7.0
B) 6.2
C) 7.5
D) 8.6
E) 7.1
A) 7.0
B) 6.2
C) 7.5
D) 8.6
E) 7.1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
A buffer is 0.282 M C6H5COOH(aq) and 0.282 M Na(C6H5COO)(aq). Calculate the pH after the addition of 0.150 moles of nitric acid to 1.0 L of the buffer. For C6H5COOH, pKa = 4.20.
A) 3.69
B) 4.20
C) 4.71
D) 3.87
A) 3.69
B) 4.20
C) 4.71
D) 3.87

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
What is the pH of a buffer solution prepared by dissolving 25.5 g NaC2H3O2 in a sufficient volume of 0.550 M HC2H3O2 to make 500.0 mL of buffer?
A) 4.74
B) 4.68
C) 4.91
D) 4.57
E) 4.79
A) 4.74
B) 4.68
C) 4.91
D) 4.57
E) 4.79

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
The buffer capacity of a solution may be defined as the number of moles of H+ that will change the pH of 1.00 L of the buffer by 1.00 pH units. What is the buffer capacity of a solution which is 0.10 M in acetic acid (Ka = 1.8 × 10-5) and 0.30 M in sodium acetate, in units of mol (H+) per liter?
A) 0.20
B) 0.10
C) 0.087
D) 0.009
E) zero
A) 0.20
B) 0.10
C) 0.087
D) 0.009
E) zero

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
An acid has a Ka = 1 × 10-6. At what pH would this acid and its corresponding salt make a good buffer?
A) 6
B) 7
C) 8
D) 5
E) 4
A) 6
B) 7
C) 8
D) 5
E) 4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
A pH 4.88 buffer was prepared by dissolving 0.10 mol of benzoic acid (Ka = 6.3 × 10-5) and 0.50 mol of sodium benzoate in sufficient pure water to form a 1.00 L solution. To a 70.0 mL aliquot of this solution was added 2.00 mL of 2.00 M HI solution. What was the pH of the new 72.0 mL solution?
A) 2.84
B) 3.16
C) 3.36
D) 4.65
E) 4.90
A) 2.84
B) 3.16
C) 3.36
D) 4.65
E) 4.90

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
What will the pH at the neutralization point of 0.00812 M Ba(OH)2 be when titrated with HCl?
A) 7.0
B) 12.2
C) 8.0
D) 9.0
E) 6.0
A) 7.0
B) 12.2
C) 8.0
D) 9.0
E) 6.0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
If 30.0 mmol HCl(g) is added to 1.00 L of a buffer that is 0.340 M NH3(aq) and 0.290 M NH4Cl(aq), what are the final concentrations of NH3(aq) and NH4Cl(aq), respectively? Assume no volume change.
A) 0.310 M and 0.320 M
B) 0.310 M and 0.290 M
C) 0.370 M and 0.290 M
D) 0.370 M and 0.320 M
A) 0.310 M and 0.320 M
B) 0.310 M and 0.290 M
C) 0.370 M and 0.290 M
D) 0.370 M and 0.320 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
What is the pH of a buffer that is 0.88 M HCN(aq) and 0.53 M NaCN(aq)? The Ka of HCN is 6.2 x 10-10.
A) 8.99
B) 9.21
C) 9.43
D) 4.79
A) 8.99
B) 9.21
C) 9.43
D) 4.79

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
A pH 9.56 buffer was prepared by mixing 2.00 moles of ammonia (Kb for ammonia is 1.8 × 10-5) and 1.00 mol of ammonium chloride in water to form a solution with a volume of 1.00 L. To a 200.0 mL aliquot of this solution was added 10.0 mL of 10.0 M sodium hydroxide. What was the resulting pH?
A) 9.28
B) 9.56
C) 9.95
D) 10.50
E) 13.7
A) 9.28
B) 9.56
C) 9.95
D) 10.50
E) 13.7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
A handbook states that to prepare a particular buffer solution mix 63.0 mL of 0.200 M HC2H3O2 with 37.0 mL of 0.200 M NaC2H3O2. What is the pH of this buffer? (Ka = 1.8 × 10-5)
A) 4.74
B) 4.51
C) 4.98
D) 5.33
E) 7.00
A) 4.74
B) 4.51
C) 4.98
D) 5.33
E) 7.00

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


