Deck 18: Solubility and Complex-Ion Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 18: Solubility and Complex-Ion Equilibria
1
The solubility of CaF2 is 0.00021 mole per liter. What is the solubility product constant for CaF2?
A) 7.3 × 10-12
B) 3.7 × 10-11
C) 8.5 × 10-8
D) 4.4 × 10-8
E) 1.9 × 10-11
A) 7.3 × 10-12
B) 3.7 × 10-11
C) 8.5 × 10-8
D) 4.4 × 10-8
E) 1.9 × 10-11
3.7 × 10-11
2
The solubility of copper(II) iodate Cu(IO3)2 is reported as 0.12 g per 100 mL. What is the solubility product constant for this salt?
A) 9.8 × 10-8
B) 8.6 × 10-7
C) 7.3 × 10-6
D) 6.4 × 10-5
E) 1.2 × 10-4
A) 9.8 × 10-8
B) 8.6 × 10-7
C) 7.3 × 10-6
D) 6.4 × 10-5
E) 1.2 × 10-4
9.8 × 10-8
3
The solubility of cerium iodate, Ce(IO3)3, molar mass = 664.83 g/mol, in pure water is 124 mg per 100 mL of water. Calculate the solubility product constant for cerium iodate.
A) 4.9 × 10-19
B) 2.6 × 10-14
C) 8.6 × 10-15
D) 3.3 × 10-10
E) 3.5 × 10-18
A) 4.9 × 10-19
B) 2.6 × 10-14
C) 8.6 × 10-15
D) 3.3 × 10-10
E) 3.5 × 10-18
3.3 × 10-10
4
Some solid in a solution at equilibrium means the solution is saturated.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
A small amount of solid calcium hydroxide is shaken vigorously in a test tube almost full of water until no further change occurs and most of the solid settles out. The resulting solution is:
A) concentrated and saturated
B) dilute and saturated
C) dilute and unsaturated
D) dilute and supersaturated
E) concentrated and supersaturated
A) concentrated and saturated
B) dilute and saturated
C) dilute and unsaturated
D) dilute and supersaturated
E) concentrated and supersaturated

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
A solute's molar solubility and its molarity are not the same in a saturated aqueous solution.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
The pH of a solution may affect solubility only if the salt contains the OH- ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Qualitative cation analysis has been replaced in recent years by instrumental analysis.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Fractional precipitation is a method to precipitate only part of the concentration of an ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
The solubility product constant of Li3PO4 is 3.2 × 10-9. What is the molar solubility of Li3PO4 in water?
A) 3.3 × 10-3 M
B) 9.3 × 10-4 M
C) 7.5 × 10-3 M
D) 1.5 × 10-3 M
E) 5.7 × 10-5 M
A) 3.3 × 10-3 M
B) 9.3 × 10-4 M
C) 7.5 × 10-3 M
D) 1.5 × 10-3 M
E) 5.7 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
The solubility of magnesium fluoride in water at 18 °C is tabulated as 0.0076 g per 100 mL. What is the solubility product for this salt?
A) 7.6 × 10-6
B) 6.8 × 10-7
C) 8.0 × 10-8
D) 7.3 × 10-9
E) 3.8 × 10-10
A) 7.6 × 10-6
B) 6.8 × 10-7
C) 8.0 × 10-8
D) 7.3 × 10-9
E) 3.8 × 10-10

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
A saturated solution of silver iodide has a concentration of 9.1 × 10-9 M. What is the Ksp of this compound?
A) 9.1 × 10-18
B) 9.1 × 10-3
C) 9.12 × 10-3
D) (2 × 9.2 × 10-3)2
E) (9.1 × 10-9)2
A) 9.1 × 10-18
B) 9.1 × 10-3
C) 9.12 × 10-3
D) (2 × 9.2 × 10-3)2
E) (9.1 × 10-9)2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
If Qsp is larger than Ksp, precipitation should occur.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
A saturated solution of magnesium fluoride has a concentration of 1.17 × 10-3 M. For this compound, Ksp =
A) 1.17 × 10-3
B) (1.17 × 10-3)2
C) 4(1.17 × 10-3)3
D) (1.17 × 10-3)3
E) 3(1.17 × 10-3)3
A) 1.17 × 10-3
B) (1.17 × 10-3)2
C) 4(1.17 × 10-3)3
D) (1.17 × 10-3)3
E) 3(1.17 × 10-3)3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
Ion pairs as a limitation to Ksp are more likely to be a problem with NaCl than with MgSO4.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
The molar solubility of SrSO4 (Ksp = 7.6 × 10-7) is:
A) 2.8 × 10-5 M
B) 7.6 × 10-7 M
C) 8.7 × 10-8 M
D) 8.7 × 10-4 M
E) 9.1 × 10-3 M
A) 2.8 × 10-5 M
B) 7.6 × 10-7 M
C) 8.7 × 10-8 M
D) 8.7 × 10-4 M
E) 9.1 × 10-3 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molar solubility of barium carbonate in pure water? (Ksp = 5.1 × 10-9)
A) 5.1 × 10-9 M
B) 7.1 × 10-5 M
C) 1.1 × 10-3
D) 1.7 × 10-3
E) 2.6 × 10-17
A) 5.1 × 10-9 M
B) 7.1 × 10-5 M
C) 1.1 × 10-3
D) 1.7 × 10-3
E) 2.6 × 10-17

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
The diverse ion effect is also called the salt effect.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
The solubility of a salt MX2 with a molar mass of 114 g/mole is 3.42 g/liter. Calculate the Ksp.
A) 2.70 × 10-5
B) 1.08 × 10-4
C) 9.00 × 10-4
D) 2.25 × 10-4
E) 6.75 × 10-8
A) 2.70 × 10-5
B) 1.08 × 10-4
C) 9.00 × 10-4
D) 2.25 × 10-4
E) 6.75 × 10-8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
The common ion effect is a case of Hess's law.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
The solubility product constant of silver sulfate is 1.6 × 10-5. What is the molar solubility of this compound?
A) 1.6 × 10-5
B) (16/2)1/2 × 10-2
C) (16/4)1/3 × 10-2
D) (16)1/2 × 10-3
E) (16/4)2/3 × 10-2
A) 1.6 × 10-5
B) (16/2)1/2 × 10-2
C) (16/4)1/3 × 10-2
D) (16)1/2 × 10-3
E) (16/4)2/3 × 10-2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following reduces the solubility of calcium fluoride?
A) presence of "uncommon ions" such as Na+ and Cl-
B) formation of the ion-pair CaF+
C) formation of the complex ion CaF42-
D) decreasing the pH
E) cooling the solution
A) presence of "uncommon ions" such as Na+ and Cl-
B) formation of the ion-pair CaF+
C) formation of the complex ion CaF42-
D) decreasing the pH
E) cooling the solution

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following should dissolve the smallest amount of silver sulfide per liter, assuming no complex formation?
A) 0.1 M HNO3
B) 0.1 M Na2S
C) 0.1 M AgNO3
D) 0.10 M NaNO3
E) pure water
A) 0.1 M HNO3
B) 0.1 M Na2S
C) 0.1 M AgNO3
D) 0.10 M NaNO3
E) pure water

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
The Ksp of AgCl is 1.7 × 10-10. How many moles of MnCl2 can be dissolved in one liter of a solution in which [AgNO3] = 3.4 × 10-4 M before a precipitate appears?
A) 5.0 × 10-7 mol
B) 5.8 × 10-14 mol
C) 2.4 × 10-7 mol
D) 2.0 × 106 mol
E) 2.5 × 10-7 mol
A) 5.0 × 10-7 mol
B) 5.8 × 10-14 mol
C) 2.4 × 10-7 mol
D) 2.0 × 106 mol
E) 2.5 × 10-7 mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
The solubility product constant of silver bromide is 5.0 × 10-13. What is the molar solubility of this compound?
A) 5.0 × 10-13
B) (50)1/2 × 10-6
C) (50)1/2 × 10-7
D) (5.0/2)1/2 × 10-7
E) (5.0 × 10-13)2
A) 5.0 × 10-13
B) (50)1/2 × 10-6
C) (50)1/2 × 10-7
D) (5.0/2)1/2 × 10-7
E) (5.0 × 10-13)2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is least soluble?
A) NiS (Ksp = 8 × 10-37)
B) MnS (Ksp = 8 × 10-16)
C) PtS (Ksp = 8 × 10-73)
D) FeS (Ksp = 4 × 10-19)
E) Hg2Cl2 (Ksp = 1.3 × 10-18)
A) NiS (Ksp = 8 × 10-37)
B) MnS (Ksp = 8 × 10-16)
C) PtS (Ksp = 8 × 10-73)
D) FeS (Ksp = 4 × 10-19)
E) Hg2Cl2 (Ksp = 1.3 × 10-18)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
The solubility product constant for iron(III) hydroxide at 22 °C is 6.0 × 10-38. What mass of this compound will dissolve in 100 mL of 0.20 M sodium hydroxide, assuming no complex formation?
A) 6 × 10-39 g
B) 7 × 10-37 g
C) 8 × 10-35 g
D) 9 × 10-33 g
E) 1 × 10-30 g
A) 6 × 10-39 g
B) 7 × 10-37 g
C) 8 × 10-35 g
D) 9 × 10-33 g
E) 1 × 10-30 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
The solubility of a salt MX2 with a molar mass of 170 g/mol is 12.7 g/liter. Calculate the Ksp.
A) 4.17 × 10-4
B) 5.58 × 10-3
C) 9.59 × 103
D) 2.23 × 10-2
E) 1.67 × 10-3
A) 4.17 × 10-4
B) 5.58 × 10-3
C) 9.59 × 103
D) 2.23 × 10-2
E) 1.67 × 10-3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Fe(OH)3 is most soluble in which solution?
A) 0.2 M HCl
B) 0.1 M KOH
C) pure water
D) 0.1 M FeCl3
E) 0.2 M CaCl2
A) 0.2 M HCl
B) 0.1 M KOH
C) pure water
D) 0.1 M FeCl3
E) 0.2 M CaCl2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
The solubility product constant of M2CrO4(s) is 7.11 × 10-15. How many moles of M2CrO4(s) will dissolve in 744 mL of solution in which [CrO42-] = 0.056 M?
A) 2.7 × 10-7 mol
B) 1.7 × 10-7 mol
C) 1.2 × 10-5 mol
D) 9.0 × 10-5 mol
E) 3.6 ⊥ 10-7 mol
A) 2.7 × 10-7 mol
B) 1.7 × 10-7 mol
C) 1.2 × 10-5 mol
D) 9.0 × 10-5 mol
E) 3.6 ⊥ 10-7 mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following has the largest molar solubility?
A) BaSO4, Ksp = 1.1 × 10-10
B) AgCl, Ksp = 1.6 × 10-10
C) Mg(OH)2, Ksp = 2 × 10-11
D) Cr(OH)2, Ksp = 6.3 × 10-11
E) Fe(OH)3, Ksp = 4 × 10-38
A) BaSO4, Ksp = 1.1 × 10-10
B) AgCl, Ksp = 1.6 × 10-10
C) Mg(OH)2, Ksp = 2 × 10-11
D) Cr(OH)2, Ksp = 6.3 × 10-11
E) Fe(OH)3, Ksp = 4 × 10-38

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the molar solubility of the following salt in a solution that contains the given concentration of one of its ions: BaSO4; [SO42-] = 4.3 × 10-5 M; Ksp = 1.1 × 10-10
A) 4.7 × 10-15 M
B) 6.9 × 10-8 M
C) 8.0 × 10-4 M
D) 2.6 × 10-6 M
E) 1.6 × 10-3 M
A) 4.7 × 10-15 M
B) 6.9 × 10-8 M
C) 8.0 × 10-4 M
D) 2.6 × 10-6 M
E) 1.6 × 10-3 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 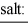
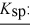 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-4 M Ca2+ + 2 × 10-2 M F-
D) 2 × 10-5 M Mg2+ + 2 × 10-6 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-
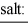
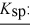 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-4 M Ca2+ + 2 × 10-2 M F-
D) 2 × 10-5 M Mg2+ + 2 × 10-6 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is most soluble?
A) CuS (Ksp = 8 × 10-37)
B) Bi2S3 (Ksp = 1 × 10-70)
C) Ag2S (Ksp = 6 × 10-51)
D) MnS (Ksp = 7 × 10-16)
E) PbS (Ksp = 3 × 10-28)
A) CuS (Ksp = 8 × 10-37)
B) Bi2S3 (Ksp = 1 × 10-70)
C) Ag2S (Ksp = 6 × 10-51)
D) MnS (Ksp = 7 × 10-16)
E) PbS (Ksp = 3 × 10-28)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following soil additives would best reduce the concentration of dissolved Fe3+ in ground water?
A) lime [Ca(OH)2]
B) saltpeter [KNO3]
C) ferric chloride [FeCl3]
D) ammonium nitrate
E) ammonium sulfate
A) lime [Ca(OH)2]
B) saltpeter [KNO3]
C) ferric chloride [FeCl3]
D) ammonium nitrate
E) ammonium sulfate

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
A saturated solution of silver chromate has a concentration of 7.4 × 10-5 M. What is the Ksp of this compound?
A) (7.4 × 10-5)2
B) (7.4 × 10-5)3
C) 3(7.4 × 10-5)3
D) 7.4 × 10-5
E) 4(7.4 × 10-5)3
A) (7.4 × 10-5)2
B) (7.4 × 10-5)3
C) 3(7.4 × 10-5)3
D) 7.4 × 10-5
E) 4(7.4 × 10-5)3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following salts, each of which has a solubility product equal to 1.0 × 10-6, has the greatest molar solubility?
A) MX
B) MX2
C) MX3
D) M2X
A) MX
B) MX2
C) MX3
D) M2X

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
Predict the molar solubility of the following salt in a solution that contains the given concentration of one of its ions: AgI; [I-] = 7.2 × 10-6 M; Ksp = 8.5 × 10-17
A) 2.4 × 10-11
B) 9.2 × 10-9
C) 1.7 × 10-6
D) 2.7 × 10-4
E) 1.2 × 10-11
A) 2.4 × 10-11
B) 9.2 × 10-9
C) 1.7 × 10-6
D) 2.7 × 10-4
E) 1.2 × 10-11

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
When solid silver chloride is shaken with a 0.1 molar solution of potassium iodide, most of the silver chloride is converted to silver iodide. This transformation takes place because:
A) silver iodide is less soluble than silver chloride
B) I- is a better reducing agent than Cl-
C) I- has a larger radius than Cl-
D) the Ksp of AgI is larger than the Ksp of AgCl
E) potassium chloride precipitates
A) silver iodide is less soluble than silver chloride
B) I- is a better reducing agent than Cl-
C) I- has a larger radius than Cl-
D) the Ksp of AgI is larger than the Ksp of AgCl
E) potassium chloride precipitates

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
What is the molar solubility of PbI2 (Ksp = 7.1 × 10-9) in 0.10 M Pb(NO3)2?
A) 1.3 × 10-4 M
B) 2.6 × 10-3 M
C) 7.1 × 10-8 M
D) 2.7 × 10-4 M
E) 2.7 × 10-5 M
A) 1.3 × 10-4 M
B) 2.6 × 10-3 M
C) 7.1 × 10-8 M
D) 2.7 × 10-4 M
E) 2.7 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
When 100 mL each of 2.0 × 10-6 M Ag+ and 2.0 × 10-3 M Br- are mixed, what is the remaining Ag+ ion concentration and is precipitation complete? The solubility product constant of AgBr is 5.0 × 10-13.
A) 7.1 × 10-7, no
B) 5.0 × 10-10, yes
C) 1.0 × 10-3, no
D) 5.0 × 10-13, yes
E) 2.5 × 10-10, yes
A) 7.1 × 10-7, no
B) 5.0 × 10-10, yes
C) 1.0 × 10-3, no
D) 5.0 × 10-13, yes
E) 2.5 × 10-10, yes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
What is the free Ag+ concentration of 0.020 M Ag+ solution mixed with an equal volume of 2.0 M NH3? Kf for [Ag(NH3)2]+ is 1.6 × 107.
A) 1.3 × 10-10
B) 2.0 × 10-14
C) 6.3 × 10-8
D) 6.5 × 10-10
E) 3.1 × 10-10
A) 1.3 × 10-10
B) 2.0 × 10-14
C) 6.3 × 10-8
D) 6.5 × 10-10
E) 3.1 × 10-10

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 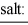
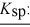 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-9
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-4 M Ca2+ + 2 × 10-4 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-5 M Mg2+ + 2 × 10-6 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-
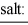
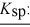 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-9
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-4 M Ca2+ + 2 × 10-4 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-5 M Mg2+ + 2 × 10-6 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
If iron(III) acetate is added to be 1 × 10-10 M in a solution that is 0.10 M NH3, what is Qsp and will Fe(OH)3 precipitate? The Ksp of Fe(OH)3 is 4 × 10-38 and Kb for NH3 is 1.8 × 10-5.
A) 1.3 × 10-13, yes
B) 1.7 × 10-16, yes
C) 1.7 × 10-16, no
D) 2.4 × 10-19, yes
E) 2.4 × 10-19, no
A) 1.3 × 10-13, yes
B) 1.7 × 10-16, yes
C) 1.7 × 10-16, no
D) 2.4 × 10-19, yes
E) 2.4 × 10-19, no

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
In which of the following one molar solutions would you expect cadmium sulfide, CdS, to be the most soluble?
A) NaCl
B) HCl
C) NaOH
D) C2H5OH
E) KOH
A) NaCl
B) HCl
C) NaOH
D) C2H5OH
E) KOH

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
When 100 mL each of 2.0 × 10-4 M Ca2+ and 2.0 × 10-2 M F- are mixed, what is the remaining Ca2+ ion concentration and is precipitation complete? The solubility product constant of CaF2 is 5.3 × 10-9.
A) 5.6 × 10-4, no
B) 1.7 × 10-8, yes
C) 4.3 × 10-7, no
D) 1.7 × 10-6, yes
E) 5.4 × 10-5, no
A) 5.6 × 10-4, no
B) 1.7 × 10-8, yes
C) 4.3 × 10-7, no
D) 1.7 × 10-6, yes
E) 5.4 × 10-5, no

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
What is the approximate concentration of free Fe3+ ion in a solution prepared by mixing equal volumes of 0.06 M Fe3+ and 4.0 M F- solutions? [The net formation constant for FeF5(H2O)2- is 2.0 × 1015.]
A) 2.8 × 10-23 M
B) 6.7 × 10-21 M
C) 6.9 × 10-19 M
D) 4.0 × 10-15 M
E) 8.9 × 10-10 M
A) 2.8 × 10-23 M
B) 6.7 × 10-21 M
C) 6.9 × 10-19 M
D) 4.0 × 10-15 M
E) 8.9 × 10-10 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
To a saturated solution of barium carbonate is added just enough sodium sulfate to achieve a maximum sulfate concentration without precipitation of barium sulfate. If no complexes form and the "salt effect" is negligible, what is the concentration of the sulfate ion in this solution? [Ksp for barium carbonate is 1.6 × 10-9; Ksp for barium sulfate is 7.9 × 10-11]
A) 2.8 × 10-5 M
B) 6.7 × 10-6 M
C) 2.0 × 10-6 M
D) 4.0 × 10-5 M
E) 8.9 × 10-5 M
A) 2.8 × 10-5 M
B) 6.7 × 10-6 M
C) 2.0 × 10-6 M
D) 4.0 × 10-5 M
E) 8.9 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Consider an aqueous solution which is 0.020 M in Pb2+ and 0.20 M in Ag+. Concentrated potassium iodide solution (so that volume changes may be neglected) is added gradually with good stirring to this solution. Eventually the iodide ion concentration should increase enough to cause precipitation of the second ion. What will be the concentration of the ion that precipitates first when the second ion just begins to precipitate?
A) 2.0 × 10-17 M
B) 1.2 × 10-8 M
C) 5.0 × 10-20 M
D) 1.4 × 10-13 M
E) 1.2 × 10-5 M
A) 2.0 × 10-17 M
B) 1.2 × 10-8 M
C) 5.0 × 10-20 M
D) 1.4 × 10-13 M
E) 1.2 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
If chromium(III) chloride is added to be 1 × 10-12 M in a solution that is 0.10 M NH3, what is Qsp and will Cr(OH)3 precipitate? The Ksp of Cr(OH)3 is 6.3 × 10-31 and Kb for NH3 is 1.8 × 10-5.
A) 1.0 × 10-16, yes
B) 2.4 × 10-23, no
C) 2.2 × 10-19, no
D) 2.2 × 10-19, yes
E) 2.4 × 10-21, yes
A) 1.0 × 10-16, yes
B) 2.4 × 10-23, no
C) 2.2 × 10-19, no
D) 2.2 × 10-19, yes
E) 2.4 × 10-21, yes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
The solubility product constant of Mg(OH)2 is 9.0 × 10-12. If a solution is 0.010 M with respect to Mg2+ ion, the amount of [OH-] required to start the precipitation of Mg(OH)2 is:
A) 1.5 × 10-7
B) 3.0 × 10-5 M
C) 3.0 × 10-7 M
D) 9.0 × 10-10
E) 1.5 × 10-5 M
A) 1.5 × 10-7
B) 3.0 × 10-5 M
C) 3.0 × 10-7 M
D) 9.0 × 10-10
E) 1.5 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
When 100 mL each of 2.0 × 10-4 M Ag+ and 2.0 × 10-1 M CO32- are mixed, what is the remaining Ag+ ion concentration and is precipitation complete? The solubility product constant of Ag2CO3 is 8.5 × 10-12.
A) 4.2 × 10-8, yes
B) 2.0 × 10-4, no
C) 9.2 × 10-6, no
D) 1.0 × 10-3, no
E) 8.5 × 10-9, yes
A) 4.2 × 10-8, yes
B) 2.0 × 10-4, no
C) 9.2 × 10-6, no
D) 1.0 × 10-3, no
E) 8.5 × 10-9, yes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
When 100 mL each of 2.0 × 10-5 M Ca2+ and 2.0 × 10-3 M CO32- are mixed, what is the remaining Ca2+ ion concentration and is precipitation complete? The solubility product constant of CaCO3 is 2.8 × 10-9.
A) 2.8 × 10-6, no
B) 1.0 × 10-3, no
C) 6.9 × 10-5, no
D) 2.8 × 10-3, no
E) 2.8 × 10-6, yes
A) 2.8 × 10-6, no
B) 1.0 × 10-3, no
C) 6.9 × 10-5, no
D) 2.8 × 10-3, no
E) 2.8 × 10-6, yes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
What is the concentration of free Zn2+ if 0.020 M Zn2+ solution is mixed with an equal volume of 2.0 M NH3? Kf for [Zn(NH3)4]2+ is 4.1 × 108.
A) 2.5 × 10-11
B) 2.9 × 10-11
C) 1.7 × 10-10
D) 9.6 × 10-11
E) 2.4 × 10-9
A) 2.5 × 10-11
B) 2.9 × 10-11
C) 1.7 × 10-10
D) 9.6 × 10-11
E) 2.4 × 10-9

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
A homeowner in Boston becomes concerned that there may be appreciable amounts of lead in her drinking water due to 150-year old water pipes in her house. Consequently, she takes a sample of her drinking water in to be analyzed. The laboratory technician, who is new on the job, has been told to analyze by precipitating the lead ion as the iodide (Ksp = 7.1 × 10-9 for PbI2) by slowly adding small portions of 1.00 M NaI solution. If we assume that the concentration of lead in the solution is 1.00 mg/liter or approximately 4.8 × 10-6 M (this would be 1.0 part per million) is it possible to detect the lead in the drinking water by adding a total of no more than 10.0 mL of NaI solution to a 100 mL sample of drinking water? You must of course consider dilution effects. What is Q?
A) yes, Q = 4.0 × 10-7
B) yes, Q = 3.6 × 10-8
C) no, Q = 1.6 × 10-13
D) no, Q = 3.6 × 10-8
E) no, Q = 4.0 × 10-7
A) yes, Q = 4.0 × 10-7
B) yes, Q = 3.6 × 10-8
C) no, Q = 1.6 × 10-13
D) no, Q = 3.6 × 10-8
E) no, Q = 4.0 × 10-7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 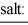
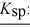 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-2 M Mg2+ + 2 × 10-3 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-
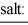
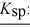 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-2 M Mg2+ + 2 × 10-3 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
A solution contains [Ba2+] = 5.0 × 10-5 M, [Ag+] = 3.0 × 10-5 M, and [Zn2+] = 2.0 × 10-7 M. Sodium oxalate is slowly added so that [C2O42-] increases. Salt BaC2O4 ZnC2O4 Ag2C2O4
Ksp 1.5 × 10-8 1.35 × 10-9 1.1 × 10-11
What is the concentration of the first cation to precipitate when the second cation just begins to precipitate?
A) 1.3 × 10-6
B) 2.2 × 10-6
C) 5.0 × 10-5
D) 1.35 × 10-9
E) 1.1 × 10-11
Ksp 1.5 × 10-8 1.35 × 10-9 1.1 × 10-11
What is the concentration of the first cation to precipitate when the second cation just begins to precipitate?
A) 1.3 × 10-6
B) 2.2 × 10-6
C) 5.0 × 10-5
D) 1.35 × 10-9
E) 1.1 × 10-11

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
To a concentrated buffer of pH 9.0 was added an equal volume of a solution that was 0.20 M in each of the ions Ca2+, Cd2+, and Cu2+. The expected precipitate would consist of: salt: calcium hydroxide cadmium hydroxide copper(II) hydroxide
Ksp: 4.0 × 10-6 2.0 × 10-14 1.8 × 10-19
A) only Ca(OH)2
B) only Cd(OH)2
C) only Cu(OH)2
D) only Cd(OH)2 and Cu(OH)2
E) Ca(OH)2, Cd(OH)2, and Cu(OH)2
Ksp: 4.0 × 10-6 2.0 × 10-14 1.8 × 10-19
A) only Ca(OH)2
B) only Cd(OH)2
C) only Cu(OH)2
D) only Cd(OH)2 and Cu(OH)2
E) Ca(OH)2, Cd(OH)2, and Cu(OH)2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
In which of the following solutions will the concentration of dissolved silver species be the highest if solid AgNO3 is stirred with 1.00 L of solution?
A) 1.00 M NH3(aq)
B) 1.00 M NaOH
C) 1.00 M Na2SO4(aq)
D) 1.00 M NaCl(aq)
E) 1.00 M NaC2H3O2(aq)
A) 1.00 M NH3(aq)
B) 1.00 M NaOH
C) 1.00 M Na2SO4(aq)
D) 1.00 M NaCl(aq)
E) 1.00 M NaC2H3O2(aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
What is the free Cu2+ concentration if 0.020 M Cu2+ solution is mixed with an equal volume of 4.0 M NH3? Kf for [Cu(NH3)4]2+ is 1.1 × 1013.
A) 5.7 × 10-15
B) 2.8 × 10-16
C) 6.2 × 10-17
D) 4.5 × 10-15
E) 1.7 × 10-16
A) 5.7 × 10-15
B) 2.8 × 10-16
C) 6.2 × 10-17
D) 4.5 × 10-15
E) 1.7 × 10-16

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
Write the solubility product constant expression for the following salt: Na2CO3.
A) [Na+][CO32-]
B) [Na+][CO32-]2
C) [Na+]2[CO32-]2
D) [Na+]2[CO32-]
E) 4[Na+]2[CO32-]
A) [Na+][CO32-]
B) [Na+][CO32-]2
C) [Na+]2[CO32-]2
D) [Na+]2[CO32-]
E) 4[Na+]2[CO32-]

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
Choose the compound that is most soluble in water.
A) K2S
B) HgS
C) ZnS
D) SnS
E) PbS
A) K2S
B) HgS
C) ZnS
D) SnS
E) PbS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
At a temperature for which the solubility product constant of calcium carbonate is 4.7 × 10-9, what mass of the salt will dissolve in 100 mL of water?
A) 0.69 mg
B) 0.94 mg
C) 1.2 mg
D) 2.4 mg
E) 4.7 mg
A) 0.69 mg
B) 0.94 mg
C) 1.2 mg
D) 2.4 mg
E) 4.7 mg

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
Equal volumes of 0.020 M Ag+ solution and 2.0 M NH3 solution are mixed. Kf for [Ag(NH3)2]+ is 1.6 × 107. If trisodium arsenate is added so that the arsenate ion concentration is 0.10 M, will silver arsenate precipitate? What is Q? Ksp for silver arsenate is 1.0 × 10-22.
A) no, Q = 6.3 × 10-9
B) no, Q = 1.9 × 10-29
C) yes, Q = 6.5 × 10-9
D) yes, Q = 4.2 × 10-20
E) no, Q = 2.6 × 10-29
A) no, Q = 6.3 × 10-9
B) no, Q = 1.9 × 10-29
C) yes, Q = 6.5 × 10-9
D) yes, Q = 4.2 × 10-20
E) no, Q = 2.6 × 10-29

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
The solubility product constant of PbI2 is 7.1 × 10-9. How many moles of PbI2 will precipitate if 250 ml of a 0.200 M solution of NaI are added to 150 ml of 0.100 M solution of Pb(NO3)2? You may neglect hydrolysis.
A) 0.050 mol
B) 1.3 × 10-5 mol
C) 0.015 mol
D) 5.6 × 10-3 mol
E) 0.040 mol
A) 0.050 mol
B) 1.3 × 10-5 mol
C) 0.015 mol
D) 5.6 × 10-3 mol
E) 0.040 mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
What is the concentration of Ca2+ in ppm (mg/L) in cave water saturated with calcite (calcium carbonate, Ksp = 4.7 × 10-9)?
A) 7.5
B) 6.9
C) 5.0
D) 3.8
E) 2.7
A) 7.5
B) 6.9
C) 5.0
D) 3.8
E) 2.7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
What is the composition of the precipitate formed when H2S gas is bubbled through 1.0 litre of a solution of 0.010 M Zn2+, 0.010 M Pb2+, and 0.010 M Mn2+, buffered at pH 2.0, until 0.10 mol of H2S has been added? [Ksp values are: ZnS: 1.6 × 10-23; MnS: 7.0 × 10-16; PbS: 7.0 × 10-29. For H2S, Ka1 = 1.0 × 10-7; Ka2 = 1.3 × 10-13.
A) only ZnS
B) only MnS
C) only PbS
D) only MnS and PbS
E) only ZnS and PbS
A) only ZnS
B) only MnS
C) only PbS
D) only MnS and PbS
E) only ZnS and PbS

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
What molar concentration of silver ion could exist in a solution in which the concentration of CrO42- is 1.0 × 10-4 M? (Ksp of Ag2CrO4 = 1.1 × 10-12)
A) 9.0 × 10-8 M
B) 4.5 × 10-3 M
C) 1.0 × 10-4 M
D) 2.1 × 10-4 M
E) 7.5 × 10-5 M
A) 9.0 × 10-8 M
B) 4.5 × 10-3 M
C) 1.0 × 10-4 M
D) 2.1 × 10-4 M
E) 7.5 × 10-5 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
Write the solubility product constant expression for the following salt: Ag2SO4.
A) [Ag+][SO42-]
B) [Ag+]2[SO42-]
C) 4[Ag+]2[SO42-]
D) [Ag+][SO42-]2
E) [Ag+]2[SO42-]2
A) [Ag+][SO42-]
B) [Ag+]2[SO42-]
C) 4[Ag+]2[SO42-]
D) [Ag+][SO42-]2
E) [Ag+]2[SO42-]2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
Write the solubility product constant for KAl(SO4)2(s)?
A) ([K+] × [Al3+] × 2[SO42-]/[KAl(SO4)2])
B) ([K+] × [Al3+] × [SO42-]/[KAl(SO4)2])
C) ([K+] × [Al3+] × [SO42-])
D) ([K+] × [Al3+] × [SO42-]2)
E) none of these
A) ([K+] × [Al3+] × 2[SO42-]/[KAl(SO4)2])
B) ([K+] × [Al3+] × [SO42-]/[KAl(SO4)2])
C) ([K+] × [Al3+] × [SO42-])
D) ([K+] × [Al3+] × [SO42-]2)
E) none of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
What is the minimum concentration of CN- that will prevent the precipitation of AgX(s) from a solution that is 0.149 M in X-(aq) and 0.0184 M in Ag+(aq)? (Ksp for AgX(s) = 5.2 × 10-17; Kf for Ag(CN)2- = 5.6 × 1018)
A) 3.1 × 10-3 M
B) 9.4 × 10-6 M
C) 1.8 × 10-10 M
D) 1.2 × 10-3 M
E) 9.6 × 10-3 M
A) 3.1 × 10-3 M
B) 9.4 × 10-6 M
C) 1.8 × 10-10 M
D) 1.2 × 10-3 M
E) 9.6 × 10-3 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Write the solubility product constant expression for the following salt: Ca3(PO4)2.
A) [Ca2+][PO43-]
B) [Ca2+]2[PO43-]3
C) [Ca2+]3[PO43-]2
D) [Ca2+]2[PO43-]
E) [Ca2+][PO43-]2
A) [Ca2+][PO43-]
B) [Ca2+]2[PO43-]3
C) [Ca2+]3[PO43-]2
D) [Ca2+]2[PO43-]
E) [Ca2+][PO43-]2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Equal volumes of a 0.020 M Zn2+ solution and a 2.0 M NH3 solution are mixed. Kf for [Zn(NH3)4]2+ is 4.1 × 108. If enough sodium oxalate is added to make the solution 0.10 M in oxalate, will ZnC2O4 precipitate? What is Q? Ksp ZnC2O4 = 2.7 × 10-8
A) yes, Q = 11
B) yes, Q = 2.9 × 10-12
C) no, Q = 2.9 × 10-12
D) yes, Q = 2.4 × 10-9
E) no, Q = 2.4 × 10-9
A) yes, Q = 11
B) yes, Q = 2.9 × 10-12
C) no, Q = 2.9 × 10-12
D) yes, Q = 2.4 × 10-9
E) no, Q = 2.4 × 10-9

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
Write the solubility product constant expression for the following salt: Ca(OH)2.
A) [Ca2+][OH-]
B) [Ca2+]2[OH-]
C) [Ca2+]3[OH-]
D) [Ca2+]2[OH-]2
E) [Ca2+][OH-]2
A) [Ca2+][OH-]
B) [Ca2+]2[OH-]
C) [Ca2+]3[OH-]
D) [Ca2+]2[OH-]2
E) [Ca2+][OH-]2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
The following table lists five compounds and their Ksp value. Which is least soluble? ZnS 2 × 10-25
TlBr 3.4 × 10-6
AgCl 1.8 × 10-10
FeS 6 × 10-19
CuI 1.1 × 10-12
A) ZnS
B) TlBr
C) AgCl
D) FeS
E) CuI
TlBr 3.4 × 10-6
AgCl 1.8 × 10-10
FeS 6 × 10-19
CuI 1.1 × 10-12
A) ZnS
B) TlBr
C) AgCl
D) FeS
E) CuI

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
When 200 mL of 0.10 M BaCl2 is added to 100 mL of 0.30 M Na2SO4, the number of moles of BaSO4 (solubility product = 1.1 × 10-10) precipitated is ________.
A) 0.020 mol
B) 0.010 mol
C) 0.20 mol
D) 0.030 mol
E) 0.10 mol
A) 0.020 mol
B) 0.010 mol
C) 0.20 mol
D) 0.030 mol
E) 0.10 mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
Write the solubility product constant expression for the following salt: PbC2O4.
A) [Pb2+][C2O42-]
B) [Pb2+]2[C2O42-]
C) [Pb2+]2[C2O42-]2
D) [Pb2+][C2O42-]2
E) 4[Pb2+][C2O42-]
A) [Pb2+][C2O42-]
B) [Pb2+]2[C2O42-]
C) [Pb2+]2[C2O42-]2
D) [Pb2+][C2O42-]2
E) 4[Pb2+][C2O42-]

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
The relationship between the molar concentration of silver ion in a saturated solution of silver phosphate and the solubility product constant for silver phosphate is ________.
A) Ksp = 9[Ag+]3
B) Ksp = 27[Ag+]4
C) Ksp = [Ag+]1/3
D) Ksp = 0.125[Ag+]
E) Ksp = 0.333[Ag+]4
A) Ksp = 9[Ag+]3
B) Ksp = 27[Ag+]4
C) Ksp = [Ag+]1/3
D) Ksp = 0.125[Ag+]
E) Ksp = 0.333[Ag+]4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
The molar solubility of calcium phosphate in a saturated aqueous solution of the salt is given by ________.
A) (Ksp/125)1/6
B) (Ksp/108)1/5
C) (Ksp/27)1/4
D) (Ksp/4)1/3
E) (Ksp)1/2
A) (Ksp/125)1/6
B) (Ksp/108)1/5
C) (Ksp/27)1/4
D) (Ksp/4)1/3
E) (Ksp)1/2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Write the solubility product constant expression for the following salt: CoS.
A) [Co2+]2[S2-]2
B) [Co2+]2[S2-]
C) [Co2+][S2-]
D) [Co2+][S2-]2
E) [Co2+]1/2[S2-]
A) [Co2+]2[S2-]2
B) [Co2+]2[S2-]
C) [Co2+][S2-]
D) [Co2+][S2-]2
E) [Co2+]1/2[S2-]

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


