Exam 18: Solubility and Complex-Ion Equilibria
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
The common ion effect is a case of Hess's law.
Free
(True/False)
4.8/5  (30)
(30)
Correct Answer:
False
Choose the compound that is most soluble in water.
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
The solubility of a salt MX2 with a molar mass of 170 g/mol is 12.7 g/liter. Calculate the Ksp.
Free
(Multiple Choice)
5.0/5  (33)
(33)
Correct Answer:
E
In which of the following one molar solutions would you expect cadmium sulfide, CdS, to be the most soluble?
(Multiple Choice)
4.8/5  (33)
(33)
In a qualitative cation analysis, the unknown ion is not precipitated by HCl, H2S, or CO32-. A flame test produced a violet flame. The unknown ion is ________.
(Multiple Choice)
4.7/5  (42)
(42)
The solubility product constant of PbI2 is 7.1 × 10-9. How many moles of PbI2 will precipitate if 250 ml of a 0.200 M solution of NaI are added to 150 ml of 0.100 M solution of Pb(NO3)2? You may neglect hydrolysis.
(Multiple Choice)
4.9/5  (34)
(34)
If chromium(III) chloride is added to be 1 × 10-12 M in a solution that is 0.10 M NH3, what is Qsp and will Cr(OH)3 precipitate? The Ksp of Cr(OH)3 is 6.3 × 10-31 and Kb for NH3 is 1.8 × 10-5.
(Multiple Choice)
4.9/5  (45)
(45)
Write the solubility product constant expression for the following salt: Ag2SO4.
(Multiple Choice)
4.9/5  (36)
(36)
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 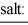
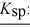 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
(Multiple Choice)
4.8/5  (41)
(41)
Saturated solutions of sodium sulfate, ammonium carbonate, and nickel (II) nitrate are mixed together. The precipitate that forms is ________.
(Multiple Choice)
4.8/5  (41)
(41)
Concentrated solutions of iron (III) chloride, sodium hydroxide, and potassium nitrate are mixed together. The precipitate which forms is ________.
(Multiple Choice)
4.8/5  (43)
(43)
What is the free Ag+ concentration of 0.020 M Ag+ solution mixed with an equal volume of 2.0 M NH3? Kf for [Ag(NH3)2]+ is 1.6 × 107.
(Multiple Choice)
4.8/5  (42)
(42)
In a qualitative cation analysis, the unknown ion is unaffected by HCl, but is precipitated by hydrogen sulfide. The unknown ion is ________.
(Multiple Choice)
4.8/5  (30)
(30)
To a concentrated buffer of pH 4.0 is added an equal volume of a solution that is 0.020 M in Cr3+, Cd2+ and Al3+. The expected precipitate would consist of ________.
salt: chromium(III) hydroxide cadmium hydroxide aluminum hydroxide
Ksp 6.3 × 10-31 2.5 × 10-14 1.3 × 10-33
(Multiple Choice)
4.9/5  (38)
(38)
In a qualitative cation analysis, the unknown ion is precipitated by HCl, remains as a chloride solid in hot water, and the precipitate turns black upon addition of ammonia. The unknown ion is ________.
(Multiple Choice)
4.9/5  (38)
(38)
A concentrated buffer of pH 8.0 is added to an equal volume of a solution that is 0.080 M in each of the ions Zn2+, Ni2+, and Mn2+. The expected precipitate would consist of ________. salt: zinc hydroxide nickel hydroxide manganese hydroxide
Ksp 1.2 × 10-17 2.0 × 10-15 1.9 × 10-13
(Multiple Choice)
4.7/5  (37)
(37)
When 100 mL each of 2.0 × 10-4 M Ca2+ and 2.0 × 10-2 M F- are mixed, what is the remaining Ca2+ ion concentration and is precipitation complete? The solubility product constant of CaF2 is 5.3 × 10-9.
(Multiple Choice)
4.8/5  (33)
(33)
At a temperature for which the solubility product constant of calcium carbonate is 4.7 × 10-9, what mass of the salt will dissolve in 100 mL of water?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)