Deck 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations
1
Which of the following is the correct "set-up" for the problem "How many grams of S are present in 29.3 g of S4N4?" ?
A)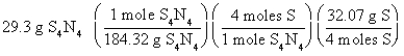
B)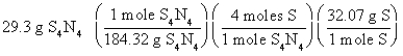
C)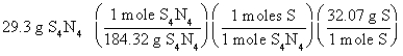
D)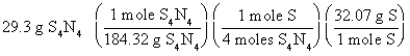
A)
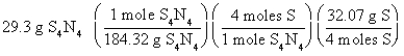
B)
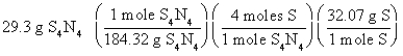
C)
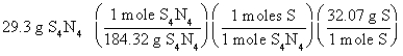
D)
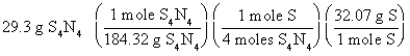
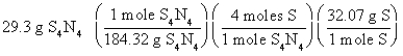
2
Which of the following samples contains the greatest number of atoms?
A) 9 mole of CO2
B) 10 moles of Xe
C) 11 moles of N2O
D) 12 moles of CO
A) 9 mole of CO2
B) 10 moles of Xe
C) 11 moles of N2O
D) 12 moles of CO
11 moles of N2O
3
Which of the following statements concerning Avogadro's number is correct?
A) It has the value 6.02*10-23.
B) It denotes the number of molecules in one mole of any molecular substance.
C) It is the mass, in grams, of one mole of any substance.
D) It denotes the number of atoms in one mole of any substance.
A) It has the value 6.02*10-23.
B) It denotes the number of molecules in one mole of any molecular substance.
C) It is the mass, in grams, of one mole of any substance.
D) It denotes the number of atoms in one mole of any substance.
It denotes the number of molecules in one mole of any molecular substance.
4
In which of the following molar quantities of sulfur would 5.95 * 1024 atoms of sulfur be present?
A) 9.69 moles S
B) 9.79 moles S
C) 9.89 moles S
D) 9.99 moles S
A) 9.69 moles S
B) 9.79 moles S
C) 9.89 moles S
D) 9.99 moles S

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
How many molecules of CO2 are present in 33.6 g of CO2?
A) 1.27 *10-24
B) 4.60 *1023
C) 8.90 *1026
D) 7.22 *1023
A) 1.27 *10-24
B) 4.60 *1023
C) 8.90 *1026
D) 7.22 *1023

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
To determine the formula mass of a compound you should:
A) Add up the atomic masses of all the atoms present.
B) Add up the atomic masses of all the atoms present and divide by the number of atoms present.
C) Add up the atomic numbers of all the atoms present.
D) Add up the atomic numbers of all the atoms present and divide by the number of atoms present.
A) Add up the atomic masses of all the atoms present.
B) Add up the atomic masses of all the atoms present and divide by the number of atoms present.
C) Add up the atomic numbers of all the atoms present.
D) Add up the atomic numbers of all the atoms present and divide by the number of atoms present.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Which set of coefficients balances the equation C4H10 + O2 CO2 + H2O?
A) 1, 6, 4, 5
B) 1, 7, 4, 5
C) 1, 3, 4, 5
D) 2, 13, 8, 10
A) 1, 6, 4, 5
B) 1, 7, 4, 5
C) 1, 3, 4, 5
D) 2, 13, 8, 10

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
In the following reaction, how many grams of H2O are produced if 6.13 g of N2H4 react? N2H4 + 3O2  2NO2 + 2H2O
2NO2 + 2H2O
A) 1.72 g
B) 3.45 g
C) 12.3 g
D) 6.89 g
 2NO2 + 2H2O
2NO2 + 2H2OA) 1.72 g
B) 3.45 g
C) 12.3 g
D) 6.89 g

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the correct "setup" for the problem "How many atoms are present in 10.4 g Ge?"
A)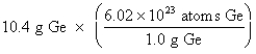
B)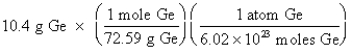
C)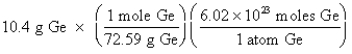
D)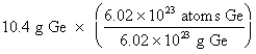
A)
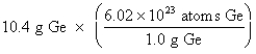
B)
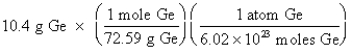
C)
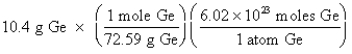
D)
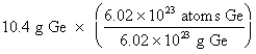

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the correct "setup" for the problem "How many grams of H2O will be produced from 2.1 moles of O2 and an excess of H2S?" according to the reaction 2H2S + 3O2  2H2O + 2SO2
2H2O + 2SO2
A)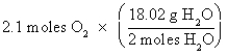
B)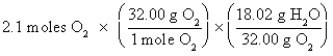
C)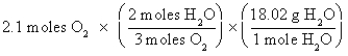
D)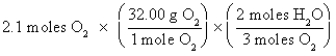
 2H2O + 2SO2
2H2O + 2SO2A)
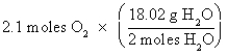
B)
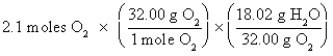
C)
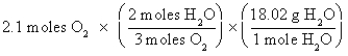
D)
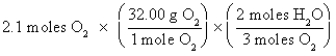

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
The formula mass of ammonium phosphite, (NH4)3PO3, is
A) 97.01 amu
B) 125.01 amu
C) 133.09 amu
D) 153.11 amu
A) 97.01 amu
B) 125.01 amu
C) 133.09 amu
D) 153.11 amu

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following samples has the largest mass, in grams?
A) 5 moles of CO2
B) 6 moles of CN
C) 7 moles of H2O
D) 8 moles of H2
A) 5 moles of CO2
B) 6 moles of CN
C) 7 moles of H2O
D) 8 moles of H2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
Avogadro's number of sulfur (S) atoms would have a mass of
A) 6.02* 10-23 g
B) 6.02 * 1023 g
C) 16
D) 32
A) 6.02* 10-23 g
B) 6.02 * 1023 g
C) 16
D) 32

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
A compound with the formula TeCln has a formula mass of 269.41 amu. What is the value for n in the formula TeCln?
A) 2
B) 3
C) 4
D) 6
A) 2
B) 3
C) 4
D) 6

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
Which of following statements concerning theoretical yield, actual yield, and percent yield is correct?
A) Theoretical yield is a calculated number.
B) Actual yield is a calculated number.
C) A percent yield of greater than 50% is very difficult to obtain.
D) Percent yield is calculated by dividing the theoretical yield by the actual yield.
A) Theoretical yield is a calculated number.
B) Actual yield is a calculated number.
C) A percent yield of greater than 50% is very difficult to obtain.
D) Percent yield is calculated by dividing the theoretical yield by the actual yield.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following chemical equations is balanced?
A) 2H2 + O2 H2O
B) 2SO2 + 2 O2 3 SO3
C) KClO3 KCl + 3 O2
D) N2 + 3H2 2NH3
A) 2H2 + O2 H2O
B) 2SO2 + 2 O2 3 SO3
C) KClO3 KCl + 3 O2
D) N2 + 3H2 2NH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
The "setup" for the problem, "What is the mass, in grams, of 5.69 *1019 atoms of Xe?" which follows is correct, except numbers in the middle conversion factor have been replaced by the letters A and B. What are the numerical values of A and B, respectively? 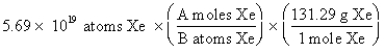
A) 1 and 6.02 * 1023
B) 1 and 131.29
C) 6.02 * 1023 and 1
D) 131.29 and 6.02 * 1023
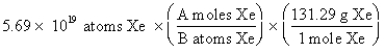
A) 1 and 6.02 * 1023
B) 1 and 131.29
C) 6.02 * 1023 and 1
D) 131.29 and 6.02 * 1023

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following conversion factors is not consistent with the equation: 4NH3 + 5 O2 4NO + 6H2O
A)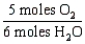
B)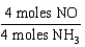
C)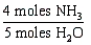
D)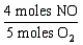
A)
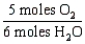
B)
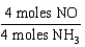
C)
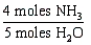
D)
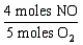

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
Potassium forms an oxide with the formula K2O. What is the coefficient of oxygen in the balanced equation for the reaction of potassium with oxygen to form this oxide?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
A mole of a chemical substance represents
A) the mass of the substance that will combine with 12.0 g of carbon
B) the mass of the substance that will combine with 100.0 g of oxygen
C) 6.02 * 1023 chemical particles of the substance
D) 6.02 * 10-23 grams of the substance
A) the mass of the substance that will combine with 12.0 g of carbon
B) the mass of the substance that will combine with 100.0 g of oxygen
C) 6.02 * 1023 chemical particles of the substance
D) 6.02 * 10-23 grams of the substance

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
The atomic masses of He and Be are 4.00 and 9.01 amu, respectively. Which of the following statements are true?
A) A mole of Be contains more atoms than a mole of He.
B) A mole of He is heavier than a mole of Be.
C) A mole of Be contains the same number of atoms as a mole of He.
D) More than one correct response.
E) No correct response.
A) A mole of Be contains more atoms than a mole of He.
B) A mole of He is heavier than a mole of Be.
C) A mole of Be contains the same number of atoms as a mole of He.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following pairings of masses does the first listed mass contain more moles of substance than the second listed mass?
A) 18.02 g H2O and 18.02 g NH3
B) 44.01 g CO2 and 44.01 g CO
C) 105 g CH4 and 105 g NO2
D) more than one correct response
E) no correct response
A) 18.02 g H2O and 18.02 g NH3
B) 44.01 g CO2 and 44.01 g CO
C) 105 g CH4 and 105 g NO2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Atoms are neither created nor destroyed in an ordinary chemical reaction.
(2) The mass of a mole of a substance depends on the identity of the substance.
(3) One mole of O2 molecules contains 6.02 * 1023 molecules.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The mass of a mole of a substance depends on the identity of the substance.
(3) One mole of O2 molecules contains 6.02 * 1023 molecules.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) 3.0 moles of CO has a greater mass, in grams, than does 2.0 moles of CO2.
(2) In a balanced chemical equation, there must be the same total number of molecules on each side of the equation.
(3) The molar mass and formula mass for a compound have different units but the same numerical value.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) In a balanced chemical equation, there must be the same total number of molecules on each side of the equation.
(3) The molar mass and formula mass for a compound have different units but the same numerical value.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
A mole of a chemical substance represents
A) the formula mass of that substance expressed in atomic mass units
B) Avogadro's number of grams of that substance
C) the mass of that substance that will combine with 1.00 gram of carbon
D) more than one correct response
E) no correct response
A) the formula mass of that substance expressed in atomic mass units
B) Avogadro's number of grams of that substance
C) the mass of that substance that will combine with 1.00 gram of carbon
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following substances has a formula mass that is greater than 80.0 amu?
A) Cl2O
B) OF2
C) SO2
D) more than one correct response
E) no correct response
A) Cl2O
B) OF2
C) SO2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following equations is balanced?
A) FeS + 2HBr FeBr2 + 2H2S
B) PbO2 + 2H2 Pb + 2H2O
C) Fe2O3 + 3H2 2Fe + 3H2O
D) more than one correct response
E) no correct response
A) FeS + 2HBr FeBr2 + 2H2S
B) PbO2 + 2H2 Pb + 2H2O
C) Fe2O3 + 3H2 2Fe + 3H2O
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is true for all balanced equations?
A) The total number of molecules on each side must be equal.
B) The sum of the subscripts on each side must be equal.
C) The sum of the coefficients on each side must be equal.
D) More than one correct response.
E) No correct response.
A) The total number of molecules on each side must be equal.
B) The sum of the subscripts on each side must be equal.
C) The sum of the coefficients on each side must be equal.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following quantities must be known before the percent yield of a product Q can be calculated?
A) theoretical yield of Q
B) actual yield of Q
C) number of other products produced besides Q
D) more than one correct response
E) no correct response
A) theoretical yield of Q
B) actual yield of Q
C) number of other products produced besides Q
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) 2.0 moles of NO contains the same number of oxygen atoms as 1.0 mole of CO2.
(2) The coefficients in a balanced equation give the fixed molar ratios between reactants and products.
(3) Avogadro's number is an experimentally determined quantity.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The coefficients in a balanced equation give the fixed molar ratios between reactants and products.
(3) Avogadro's number is an experimentally determined quantity.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
One mole of H2SO4 contains
A) two moles H atoms
B) four moles S atoms
C) seven moles of atoms
D) more than one correct response
E) no correct response
A) two moles H atoms
B) four moles S atoms
C) seven moles of atoms
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
The balanced equation C + O2 CO2 tells us that 24.02 g of C will exactly react with
A) 32.00 g of O2
B) 2.000 moles of O2
C) 6.02 * 1023 molecules of O2
D) more than one correct response
E) no correct response
A) 32.00 g of O2
B) 2.000 moles of O2
C) 6.02 * 1023 molecules of O2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
The balanced equation 2CO + O2 2CO2 tells us that:
A) One gram of O2 will produce two grams of CO2.
B) Two molecules of CO will react with one molecule of O2.
C) One mole of CO will produce two moles of CO2.
D) More than one correct response.
E) No correct response.
A) One gram of O2 will produce two grams of CO2.
B) Two molecules of CO will react with one molecule of O2.
C) One mole of CO will produce two moles of CO2.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
For which of the following compounds does 1.9 g represent 4.3 * 10-2 moles of compound?
A) CO2
B) C3H8
C) H2O2
D) more than one correct response
E) no correct response
A) CO2
B) C3H8
C) H2O2
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The molar mass of an element, in the solid state, has the same numerical value as the element's atomic mass.
(2) In a balanced chemical equation, the number of reactants must equal the number of products.
(3) One mole of N2O4 contains six moles of atoms.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) In a balanced chemical equation, the number of reactants must equal the number of products.
(3) One mole of N2O4 contains six moles of atoms.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) In the notation 2H2O, the 2 out in front is called a coefficient.
(2) Atomic masses and formula masses are specified using the same units.
(3) In a formula-based "grams of A to grams of B" problem, molar masses are needed in each of the first two conversion factors.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Atomic masses and formula masses are specified using the same units.
(3) In a formula-based "grams of A to grams of B" problem, molar masses are needed in each of the first two conversion factors.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
In which of the following groups of substances do all members of the group have atomic masses that are within 1.00 amu of each other?
A) CH4, NH3, H2O
B) CO, N2, NO
C) C3H8, CO2, N2O
D) more than one correct response
E) no correct response
A) CH4, NH3, H2O
B) CO, N2, NO
C) C3H8, CO2, N2O
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following samples has a mass greater than 50.00 grams?
A) 6.02 *1023 atoms of Fe
B) 3.01 * 1023 molecules of CH4
C) 9.03 * 1023 molecules of H2O
D) more than one correct response
E) no correct response
A) 6.02 *1023 atoms of Fe
B) 3.01 * 1023 molecules of CH4
C) 9.03 * 1023 molecules of H2O
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
Avogadro's number is equal to the number of
A) atoms in one mole of N2
B) molecules in one mole of N2O4
C) atoms in one-half mole of CO
D) more than one correct response
E) no correct response
A) atoms in one mole of N2
B) molecules in one mole of N2O4
C) atoms in one-half mole of CO
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following unbalanced equations are five moles of reactants required to produce two moles of products?
A) Na + N2 NaN3
B) Al + S Al2S3
C) N2 + H2 NH3
D) more than one correct response
E) no correct response
A) Na + N2 NaN3
B) Al + S Al2S3
C) N2 + H2 NH3
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Select the correct numerical value for the mass, in grams, of 6.02 * 1023 atoms of C.
A) 12.0
B) 24.0
C) 44.0
D) 88.0
A) 12.0
B) 24.0
C) 44.0
D) 88.0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Select the correct numerical value for the number of moles of S in two moles of H2SO4.
A) 1
B) 2
C) 6
D) 12
A) 1
B) 2
C) 6
D) 12

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Six mole-to-mole relationships are obtainable from a balanced chemical equation involving two reactants and two products.
(2) The compound H2O has a greater formula mass than does the compound NH3.
(3) The atomic mass unit (amu) and the gram unit (g) are related to one another through Avogadro's number.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The compound H2O has a greater formula mass than does the compound NH3.
(3) The atomic mass unit (amu) and the gram unit (g) are related to one another through Avogadro's number.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Contrast the magnitudes, to three significant figures, (I) atoms in three moles of CO, (II) atoms in two moles of CO2, and then select an appropriate response from the response list.
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Select the correct numerical value for the atomic mass, in amu, of C.
A) 12.0
B) 24.0
C) 44.0
D) 88.0
A) 12.0
B) 24.0
C) 44.0
D) 88.0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Two moles of O2 molecules contain twice Avogadro's number of atoms.
(2) The actual yield of a product in a chemical reaction is a measured rather than calculated quantity.
(3) In an equation-based "grams of A to grams of B" problem, equation coefficients become part of the first needed conversion factor.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The actual yield of a product in a chemical reaction is a measured rather than calculated quantity.
(3) In an equation-based "grams of A to grams of B" problem, equation coefficients become part of the first needed conversion factor.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Avogadro's number is part of the conversion factor needed to solve a "moles of A to grams of A" problem.
(2) The sum of the coefficients in the balanced equation for the production of water from hydrogen gas and oxygen gas is five.
(3) 2.00 moles of NH3 molecules have a mass of 34.0 grams.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The sum of the coefficients in the balanced equation for the production of water from hydrogen gas and oxygen gas is five.
(3) 2.00 moles of NH3 molecules have a mass of 34.0 grams.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Select the correct numerical value for the molar mass, in grams, of CO2.
A) 12.0
B) 24.0
C) 44.0
D) 88.0
A) 12.0
B) 24.0
C) 44.0
D) 88.0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) In balancing an equation, formula subscripts are adjusted as needed to obtain the balance.
(2) 1.00 mole of C contains the same number of atoms as does 12.0 g of C.
(3) Both a microscopic and macroscopic levels of interpretation exist for a chemical formula with the former involving atoms and the latter involving molecules.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) 1.00 mole of C contains the same number of atoms as does 12.0 g of C.
(3) Both a microscopic and macroscopic levels of interpretation exist for a chemical formula with the former involving atoms and the latter involving molecules.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Select the correct numerical value for the number of moles of atoms in two moles of SO2.
A) 1
B) 2
C) 6
D) 12
A) 1
B) 2
C) 6
D) 12

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Contrast the magnitudes, to three significant figures, (I) molecules in 28.0 g of N2, (II) molecules in 28.0 g of O2, and then select an appropriate response from the response list.
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Select the correct numerical value for the number of moles of H in one mole of (NH4)3PO4.
A) 1
B) 2
C) 6
D) 12
A) 1
B) 2
C) 6
D) 12

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Carbon monoxide reacts with hemoglobin in red blood cells to produce carbon dioxide, which changes blood's acidity.
(2) Sulfuric acid is the number two chemical in the United States in terms of production amount.
(3) The raw materials needed for industrial sulfuric acid production are sulfur, air and water.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Sulfuric acid is the number two chemical in the United States in terms of production amount.
(3) The raw materials needed for industrial sulfuric acid production are sulfur, air and water.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Contrast the magnitudes, to three significant figures, (I) moles in 28.0 g of N2, (II) moles in 28.0 g of CO, and then select an appropriate response from the response list.
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Select the correct numerical value for the formula mass, in amu, of CO2.
A) 12.0
B) 24.0
C) 44.0
D) 88.0
A) 12.0
B) 24.0
C) 44.0
D) 88.0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
Select the correct numerical value for the number of moles of O atoms in three moles of O2
A) 1
B) 2
C) 6
D) 12
A) 1
B) 2
C) 6
D) 12

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Contrast the magnitudes, to three significant figures, (I) molecules in two moles of CO, (II) molecules in two moles of CO2, and then select an appropriate response from the response list.
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Select the correct numerical value for the number of moles of O2 molecules in two moles of O2.
A) 1
B) 2
C) 6
D) 12
A) 1
B) 2
C) 6
D) 12

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Contrast the magnitudes, to three significant figures, (I) mass of one mole of CO2, (II) the mass of one mole of NH3, and then select an appropriate response from the response list.
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information
A) I is greater than II
B) I is equal to II
C) I is less than II
D) I and II cannot be compared because of insufficient information

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Select the correct numerical value for the mass, in grams, of 2.00 moles of CO2
A) 12.0
B) 24.0
C) 44.0
D) 88.0
A) 12.0
B) 24.0
C) 44.0
D) 88.0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Select the set of coefficients from the response list that correctly balances the equation. __H2 + N2 NH3
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many moles of O2 needed to produce 12 moles of H2O?
A) 6
B) 7
C) 8
D) 14
A) 6
B) 7
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Select the set of coefficients from the response list that correctly balances the equation. __SO2 + O2 SO3
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Select the set of coefficients from the response list that correctly balances the equation. __N2 + O2 NO
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many molecules of CO2 produced when four molecules of C2H6 react?
A) 6
B) 7
C) 8
D) 14
A) 6
B) 7
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Select the set of coefficients from the response list that correctly balances the equation. __CO + O2 CO2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many moles of O2 that react with four moles of C2H6?
A) 6
B) 7
C) 8
D) 14
A) 6
B) 7
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many molecules of O2 that react with two molecules of C2H6?
A) 6
B) 7
C) 8
D) 14
A) 6
B) 7
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many moles of CO2 produced at the same time nine moles of H2O are produced?
A) 6
B) 7
C) 8
D) 14
A) 6
B) 7
C) 8
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Select the set of coefficients from the response list that correctly balances the equation. __CH4 __C3H8 + __H2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2
A) 1, 1, 2
B) 1, 2, 1
C) 2, 1, 2
D) 3, 1, 2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


