Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
Select the set of coefficients from the response list that correctly balances the equation. __CH4 __C3H8 + __H2
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
D
Which of the following is the correct "setup" for the problem "How many grams of H2O will be produced from 2.1 moles of O2 and an excess of H2S?" according to the reaction 2H2S + 3O2  2H2O + 2SO2
2H2O + 2SO2
Free
(Multiple Choice)
4.7/5  (31)
(31)
Correct Answer:
C
To determine the formula mass of a compound you should:
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
A
Which set of coefficients balances the equation C4H10 + O2 CO2 + H2O?
(Multiple Choice)
4.9/5  (32)
(32)
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) In balancing an equation, formula subscripts are adjusted as needed to obtain the balance.
(2) 1.00 mole of C contains the same number of atoms as does 12.0 g of C.
(3) Both a microscopic and macroscopic levels of interpretation exist for a chemical formula with the former involving atoms and the latter involving molecules.
(Multiple Choice)
4.8/5  (39)
(39)
In which of the following unbalanced equations are five moles of reactants required to produce two moles of products?
(Multiple Choice)
4.9/5  (32)
(32)
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Avogadro's number is part of the conversion factor needed to solve a "moles of A to grams of A" problem.
(2) The sum of the coefficients in the balanced equation for the production of water from hydrogen gas and oxygen gas is five.
(3) 2.00 moles of NH3 molecules have a mass of 34.0 grams.
(Multiple Choice)
4.9/5  (37)
(37)
Which of following statements concerning theoretical yield, actual yield, and percent yield is correct?
(Multiple Choice)
4.8/5  (38)
(38)
Select the correct numerical value for the number of moles of atoms in two moles of SO2.
(Multiple Choice)
4.9/5  (38)
(38)
A compound with the formula TeCln has a formula mass of 269.41 amu. What is the value for n in the formula TeCln?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following quantities must be known before the percent yield of a product Q can be calculated?
(Multiple Choice)
4.8/5  (45)
(45)
Select the set of coefficients from the response list that correctly balances the equation. __CO + O2 CO2
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements concerning Avogadro's number is correct?
(Multiple Choice)
4.9/5  (27)
(27)
Using the balanced equation for the combustion of ethane: 2C2H6 + 7 O2 4CO2 + 6H2O, how many moles of CO2 produced at the same time nine moles of H2O are produced?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following samples has the largest mass, in grams?
(Multiple Choice)
4.7/5  (27)
(27)
The "setup" for the problem, "What is the mass, in grams, of 5.69 1019 atoms of Xe?" which follows is correct, except numbers in the middle conversion factor have been replaced by the letters A and B. What are the numerical values of A and B, respectively? 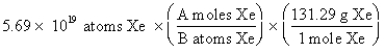
(Multiple Choice)
4.8/5  (35)
(35)
In which of the following pairings of masses does the first listed mass contain more moles of substance than the second listed mass?
(Multiple Choice)
4.9/5  (35)
(35)
Select the set of coefficients from the response list that correctly balances the equation. __N2 + O2 NO
(Multiple Choice)
4.9/5  (27)
(27)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)