Deck 2: Measurements in Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 2: Measurements in Chemistry
1
Which of the following would involve an exact number?
A) the length of a table
B) the mass of a bag of carrots
C) the number of inches in a yard
D) the surface area of a quilt
A) the length of a table
B) the mass of a bag of carrots
C) the number of inches in a yard
D) the surface area of a quilt
the number of inches in a yard
2
According to dimensional analysis, which of the following is the correct setup for the problem "How many milligrams are there in 85 kilograms?"
A) 85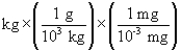
B) 85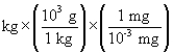
C) 85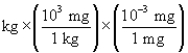
D) 85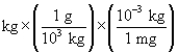
A) 85
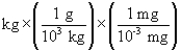
B) 85
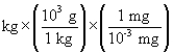
C) 85
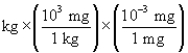
D) 85
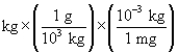
85 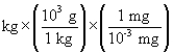
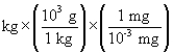
3
If the temperature of an object is 435 oC, what is the temperature on a Kelvin scale?
A) 162 K
B) 608 K
C) 672 K
D) 708 K
A) 162 K
B) 608 K
C) 672 K
D) 708 K
708 K
4
How many conversion factors can be derived from the equality 60 seconds = 1 minute?
A) two
B) three
C) four
D) an unlimited number
A) two
B) three
C) four
D) an unlimited number

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
In which of the following pairs of measured numbers does each member of the pair contain the same number of significant figures?
A) 10.3 and 10.30
B) 800.0 and 80
C) 0.03330 and 0.0333
D) 0.000096 and 960,000
A) 10.3 and 10.30
B) 800.0 and 80
C) 0.03330 and 0.0333
D) 0.000096 and 960,000

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
The correct answer obtained from adding the measurements 9.6, 4.79, and 5.352 contains
A) two significant figures
B) three significant figures
C) four significant figures
D) five significant figures
A) two significant figures
B) three significant figures
C) four significant figures
D) five significant figures

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
In which one of the following measure numbers are all of the zeros significant?
A) 0.0705
B) 3,300,000
C) 0.000440
D) 3.945900
A) 0.0705
B) 3,300,000
C) 0.000440
D) 3.945900

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
The correct answer obtained by dividing the measurement 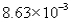 by the measurement is
by the measurement is
A)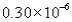
B)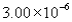
C)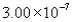
D)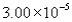
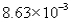 by the measurement is
by the measurement isA)
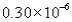
B)
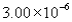
C)
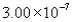
D)
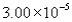

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following cases is the given measurement correctly rounded to three significant figures?
A) 479,000 becomes 479
B) 0.02235 becomes 0.0223
C) 37.98 becomes 38.0
D) 49.400 becomes 49,400
A) 479,000 becomes 479
B) 0.02235 becomes 0.0223
C) 37.98 becomes 38.0
D) 49.400 becomes 49,400

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
The measurement 8310.90 expressed in scientific notation becomes
A)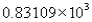
B)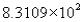
C)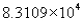
D)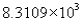
A)
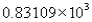
B)
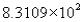
C)
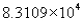
D)
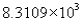

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
What is the uncertainty associated with the measurement of 6.02 x 104?
A) 100
B) 10
C) 0.1
D) 0.01
A) 100
B) 10
C) 0.1
D) 0.01

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
The density of an object is the ratio of its
A) length to volume
B) mass to height
C) mass to volume
D) length to mass
A) length to volume
B) mass to height
C) mass to volume
D) length to mass

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following comparisons of the size of a degree on the major temperature scales is correct?
A) A Kelvin degree is larger than a Celsius degree.
B) A Fahrenheit degree and a Celsius degree are equal in size.
C) A Fahrenheit degree is larger than a Kelvin degree.
D) A Celsius degree and a Kelvin degree are equal in size.
A) A Kelvin degree is larger than a Celsius degree.
B) A Fahrenheit degree and a Celsius degree are equal in size.
C) A Fahrenheit degree is larger than a Kelvin degree.
D) A Celsius degree and a Kelvin degree are equal in size.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
The calculator answer obtained from multiplying the measurements 64.49 and 6.57 is 423.70. Given the operational rules governing significant figures, this answer
A) is correct as written
B) should be rounded to 423.7
C) should be rounded to 424
D) could be written as
A) is correct as written
B) should be rounded to 423.7
C) should be rounded to 424
D) could be written as


Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
If object A weighs 6.0 grams and has a volume of 3.0 mL and object B weighs 9.0 grams and has a volume of 2.25 mL
A) B is less dense than A.
B) A and B have equal densities.
C) B is twice as dense as A.
D) B is four times as dense as A.
A) B is less dense than A.
B) A and B have equal densities.
C) B is twice as dense as A.
D) B is four times as dense as A.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
In which of the following sequences are the metric system prefixes listed in order of decreasing size?
A) kilo giga mega
B) milli nano micro
C) mega kilo micro
D) pico kilo deci
A) kilo giga mega
B) milli nano micro
C) mega kilo micro
D) pico kilo deci

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
To what decimal position should a measurement be recorded if the smallest markings on the measurement scale are tenths of a centimeter?
A) to the closest centimeter
B) to the tenths of a centimeter
C) to the hundredths of a centimeter
D) to the thousandths of a centimeter
A) to the closest centimeter
B) to the tenths of a centimeter
C) to the hundredths of a centimeter
D) to the thousandths of a centimeter

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
The "mathematical meaning" associated with the metric system prefixes centi, milli, and micro is, respectively,
A) 10-2, 10-4, and 10-6
B) 10-2, 10-3, and 10-6
C) 10-3, 10-6, and 10-9
D) 10-3, 10-9, and 10-12
A) 10-2, 10-4, and 10-6
B) 10-2, 10-3, and 10-6
C) 10-3, 10-6, and 10-9
D) 10-3, 10-9, and 10-12

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
What is the mass, in grams, of 30.7 mL of a liquid if its density is 0.81 g/mL?
A) 3.8
B) 25
C) 4
D) 249
A) 3.8
B) 25
C) 4
D) 249

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an incorrect pairing of terminology?
A) kilogram - metric unit of mass
B) milliliter - metric unit of volume
C) meter - metric unit of length
D) cubic centimeter - metric unit of length
A) kilogram - metric unit of mass
B) milliliter - metric unit of volume
C) meter - metric unit of length
D) cubic centimeter - metric unit of length

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Statements:
(1) The answer to the addition problem 3.21 + 32 + 3.22 should have an uncertainty of hundredths.
(2) The measurement 653,899, when rounded to five significant figures, becomes 65,390.
(3) The higher the specific heat of a substance, the more its temperature will change when it absorbs a given amount of heat.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(1) The answer to the addition problem 3.21 + 32 + 3.22 should have an uncertainty of hundredths.
(2) The measurement 653,899, when rounded to five significant figures, becomes 65,390.
(3) The higher the specific heat of a substance, the more its temperature will change when it absorbs a given amount of heat.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following measured numbers contains three significant figures and has a magnitude of less than one?
A) 3.30* 105
B) 3.00 *10-3
C) 3.20 *10-4
D) more than one correct response
E) no correct response
A) 3.30* 105
B) 3.00 *10-3
C) 3.20 *10-4
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements concerning conversion factors is incorrect?
A) English-to-English conversion factors come from defined relationships
B) Metric-to-metric conversions come from measured relationships
C) English-to-English conversion factors always contain exact numbers
D) more than one correct response
E) no correct response
A) English-to-English conversion factors come from defined relationships
B) Metric-to-metric conversions come from measured relationships
C) English-to-English conversion factors always contain exact numbers
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
When expressed in scientific notation, the measured numbers 3200 and 3200.0 become, respectively,
A) 3.2 *103 and 3.200*103
B) 3.2*103 and 3.2000 *103
C) 3.200 * 103 and 3.2000*103
D) more than one correct response
E) no correct response
A) 3.2 *103 and 3.200*103
B) 3.2*103 and 3.2000 *103
C) 3.200 * 103 and 3.2000*103
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following digits in the measurement 654,300 seconds is an estimated digit?
A) the last digit
B) the next to last zero
C) the three
D) more than one correct response
E) no correct response
A) the last digit
B) the next to last zero
C) the three
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
Density can be used as a conversion factor to convert from
A) mass to volume
B) volume to mass
C) metric unit mass to English unit mass
D) more than one correct response
E) no correct response
A) mass to volume
B) volume to mass
C) metric unit mass to English unit mass
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Statements:
(1) The meaning of a metric system prefix is independent of the base unit it modifies.
(2) "Trailing zeros" at the end of a measured number are never significant.
(3) The answer to the problem 105/10-3 is 102.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(1) The meaning of a metric system prefix is independent of the base unit it modifies.
(2) "Trailing zeros" at the end of a measured number are never significant.
(3) The answer to the problem 105/10-3 is 102.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements concerning the three major temperature scales is correct?
A) Kelvin temperatures are always positive.
B) The equation for converting from Celsius to Kelvin involves the number 273.
C) The freezing point of water has a lower numerical value on the Kelvin scale than on the Fahrenheit scale.
D) More than one correct response.
E) No correct response.
A) Kelvin temperatures are always positive.
B) The equation for converting from Celsius to Kelvin involves the number 273.
C) The freezing point of water has a lower numerical value on the Kelvin scale than on the Fahrenheit scale.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements concerning the measured number 0.3030 is correct?
A) Only one of the zeros in the number is significant.
B) Rounded off to two significant figures the number becomes 0.30.
C) Expressed in scientific notation the number becomes 3.03 *10-1.
D) More than one correct response.
E) No correct response.
A) Only one of the zeros in the number is significant.
B) Rounded off to two significant figures the number becomes 0.30.
C) Expressed in scientific notation the number becomes 3.03 *10-1.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
In which of the following sequences of measured numbers do all members of the sequence contain three significant figures?
A) 3.03 and 3.30 and 0.033
B) 78,000 and 0.00780 and 780
C) 30.0 and 0.300 and 30,100
D) more than one correct response
E) no correct response
A) 3.03 and 3.30 and 0.033
B) 78,000 and 0.00780 and 780
C) 30.0 and 0.300 and 30,100
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Statements: (1) In outer space, an astronaut may be weightless but never massless.
(2) The metric system prefixes milli and micro differ in mathematical meaning by a factor of 1000.
(3) The addition of 273 to a Fahrenheit temperature reading will convert it to a Kelvin temperature reading.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The metric system prefixes milli and micro differ in mathematical meaning by a factor of 1000.
(3) The addition of 273 to a Fahrenheit temperature reading will convert it to a Kelvin temperature reading.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following conversion factors would limit a calculation to two significant figures?
A)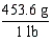
B)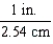
C)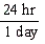
D) more than one correct response
E) no correct response
A)
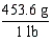
B)
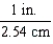
C)
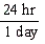
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
In which of the following pairs of units is the first listed unit 1000 times larger than the second?
A) milligram and nanogram
B) liter and centiliter
C) kilometer and megameter
D) more than one correct response
E) no correct response
A) milligram and nanogram
B) liter and centiliter
C) kilometer and megameter
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following measured numbers has an uncertainty of 0.01 associated with it?
A) 32.930
B) 3.02 * 106
C) 3.0 *10-1
D) more than one correct response
E) no correct response
A) 32.930
B) 3.02 * 106
C) 3.0 *10-1
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following mathematical expressions is correctly evaluated?
A)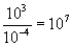
B) 103 * 104 = 1012
C)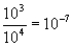
D) more than one correct response
E) no correct response
A)
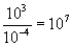
B) 103 * 104 = 1012
C)
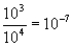
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
In which of the following pairs of temperature readings are the two members of the pair equivalent to each other?
A) 32 F and 273 K
B) 0 C and 373 K
C) 0 C and 40 F
D) more than one correct response
E) no correct response
A) 32 F and 273 K
B) 0 C and 373 K
C) 0 C and 40 F
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Statements: (1) The measured number 2.410 * 10-3 contains three significant figures.
(2) The specific heat of water is higher than that of most other substances.
(3) The equation 1 kg = 106 mg is a correct mathematical statement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The specific heat of water is higher than that of most other substances.
(3) The equation 1 kg = 106 mg is a correct mathematical statement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
The density of table sugar is 1.59 g/mL. It is true that
A) 2.00 g of table sugar occupies a volume of 1.17 mL.
B) 3.00 g of table sugar occupies a volume of 1.97 mL.
C) 5.00 g of table sugar occupies a volume of 3.14 mL.
D) More than one correct response
E) No correct response
A) 2.00 g of table sugar occupies a volume of 1.17 mL.
B) 3.00 g of table sugar occupies a volume of 1.97 mL.
C) 5.00 g of table sugar occupies a volume of 3.14 mL.
D) More than one correct response
E) No correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Statements: (1) The conversion factor 103 m/1 km contains an unlimited number of significant figures.
(2) Density may be used as a conversion factor to convert from mass to volume.
(3) The equation 2.33 lb = 625 g is a correct mathematical statement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Density may be used as a conversion factor to convert from mass to volume.
(3) The equation 2.33 lb = 625 g is a correct mathematical statement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following pairings of metric system prefix and power of ten is the pairing incorrect?
A) kilo- and 10-3
B) micro- and 10-6
C) deci- and 101
D) more than one correct response
E) no correct response
A) kilo- and 10-3
B) micro- and 10-6
C) deci- and 101
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Statements: (1) A deciliter is equal to 100 milliliters.
(2) The Kelvin temperature scale is closely related mathematically to the Celsius temperature scale.
(3) Measurements cannot be exact because two estimated digits are always recorded as part of any measurement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The Kelvin temperature scale is closely related mathematically to the Celsius temperature scale.
(3) Measurements cannot be exact because two estimated digits are always recorded as part of any measurement.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Statements:
(1) The answer to the calculation 12.00 *(6.00 * 1023) should contain three significant figures.
(2) A meter is slightly larger than a yard, and a liter is slightly larger than a quart.
(3) The numbers 3.30 * 10-1 and 3.30 * 101 both have a magnitude of less than one.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(1) The answer to the calculation 12.00 *(6.00 * 1023) should contain three significant figures.
(2) A meter is slightly larger than a yard, and a liter is slightly larger than a quart.
(3) The numbers 3.30 * 10-1 and 3.30 * 101 both have a magnitude of less than one.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Statements: (1) The measured numbers 244,000 and 0.000244 contain the same number of significant figures.
(2) One cubic centimeter is equal to ten milliliters.
(3) The conversion factor 1 in/2.54 cm, when used as written, would decrease unit size.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) One cubic centimeter is equal to ten milliliters.
(3) The conversion factor 1 in/2.54 cm, when used as written, would decrease unit size.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Statements:
(1) The size of the degree is the same on the Fahrenheit and Celsius temperature scales.
(2) The measurement 62,300 has an uncertainty of 100.
(3) The answer to the calculation 8.45 + 10.40 should contain four significant figures.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(1) The size of the degree is the same on the Fahrenheit and Celsius temperature scales.
(2) The measurement 62,300 has an uncertainty of 100.
(3) The answer to the calculation 8.45 + 10.40 should contain four significant figures.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck


