Exam 2: Measurements in Chemistry
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
What is the uncertainty associated with the measurement of 6.02 x 104?
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
A
Statements: (1) The measured number 2.410 10-3 contains three significant figures.
(2) The specific heat of water is higher than that of most other substances.
(3) The equation 1 kg = 106 mg is a correct mathematical statement.
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
B
Which of the following statements concerning the three major temperature scales is correct?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
D
The correct answer obtained by dividing the measurement 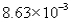 by the measurement is
by the measurement is
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following measured numbers contains three significant figures and has a magnitude of less than one?
(Multiple Choice)
4.8/5  (28)
(28)
To what decimal position should a measurement be recorded if the smallest markings on the measurement scale are tenths of a centimeter?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements concerning the measured number 0.3030 is correct?
(Multiple Choice)
4.9/5  (34)
(34)
In which of the following pairings of metric system prefix and power of ten is the pairing incorrect?
(Multiple Choice)
4.8/5  (32)
(32)
Statements:
(1) The meaning of a metric system prefix is independent of the base unit it modifies.
(2) "Trailing zeros" at the end of a measured number are never significant.
(3) The answer to the problem 105/10-3 is 102.
(Multiple Choice)
4.9/5  (33)
(33)
The measurement 8310.90 expressed in scientific notation becomes
(Multiple Choice)
4.7/5  (29)
(29)
The "mathematical meaning" associated with the metric system prefixes centi, milli, and micro is, respectively,
(Multiple Choice)
4.8/5  (32)
(32)
Statements:
(1) The answer to the calculation 12.00 (6.00 1023) should contain three significant figures.
(2) A meter is slightly larger than a yard, and a liter is slightly larger than a quart.
(3) The numbers 3.30 10-1 and 3.30 101 both have a magnitude of less than one.
(Multiple Choice)
5.0/5  (33)
(33)
In which of the following pairs of temperature readings are the two members of the pair equivalent to each other?
(Multiple Choice)
4.9/5  (36)
(36)
In which of the following sequences are the metric system prefixes listed in order of decreasing size?
(Multiple Choice)
5.0/5  (38)
(38)
What is the mass, in grams, of 30.7 mL of a liquid if its density is 0.81 g/mL?
(Multiple Choice)
4.8/5  (41)
(41)
In which of the following pairs of measured numbers does each member of the pair contain the same number of significant figures?
(Multiple Choice)
4.8/5  (35)
(35)
Statements: (1) The conversion factor 103 m/1 km contains an unlimited number of significant figures.
(2) Density may be used as a conversion factor to convert from mass to volume.
(3) The equation 2.33 lb = 625 g is a correct mathematical statement.
(Multiple Choice)
4.7/5  (40)
(40)
When expressed in scientific notation, the measured numbers 3200 and 3200.0 become, respectively,
(Multiple Choice)
5.0/5  (39)
(39)
In which of the following pairs of units is the first listed unit 1000 times larger than the second?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 1 - 20 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)