Deck 3: Ionic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 3: Ionic Compounds
1
Which is not a covalent compound?
A)Br2
B)NO2
C)NH3
D)NaNO2
E)More than one of the compounds above is not a covalent compound.
A)Br2
B)NO2
C)NH3
D)NaNO2
E)More than one of the compounds above is not a covalent compound.
D
2
In bonding, main group elements _____ to attain the electronic configuration of the noble gas closest to them in the periodic table.
A)gain electrons
B)lose electrons
C)share electrons
D)gain, lose, or share electrons
A)gain electrons
B)lose electrons
C)share electrons
D)gain, lose, or share electrons
D
3
Which is not an ionic compound?
A)KCl
B)Ca(NO3)2
C)CBr4
D)AlBr3
A)KCl
B)Ca(NO3)2
C)CBr4
D)AlBr3
C
4
What is the formula for the ionic compound formed when aluminum and sulfur combine?
A)AlS
B)Al2S3
C)AlS3
D)Al2S
E)Al3S2
A)AlS
B)Al2S3
C)AlS3
D)Al2S
E)Al3S2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
_____ are negatively charged ions that have _____ electrons than protons.
A)Anions, more
B)Cations, more
C)Anions, less
D)Cations, less
A)Anions, more
B)Cations, more
C)Anions, less
D)Cations, less

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
What is the formula for the ionic compound formed when magnesium and oxygen combine?
A)MgO
B)MgO2
C)Mg2O
D)Mg2O2
A)MgO
B)MgO2
C)Mg2O
D)Mg2O2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
What is the symbol for the ion formed by magnesium?
A)Mg2-
B)Mg3+
C)Mg2+
D)Mg4-
A)Mg2-
B)Mg3+
C)Mg2+
D)Mg4-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Which element has a variable charge?
A)Na
B)Fe
C)Al
D)C
A)Na
B)Fe
C)Al
D)C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
What is the ion symbol of the oxide ion?
A)O2-
B)O22-
C)O2
D)O2+
E)O6-
A)O2-
B)O22-
C)O2
D)O2+
E)O6-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
What is the formula for the ionic compound formed when potassium and bromine combine?
A)PBr
B)KBr
C)KBr2
D)K2Br
E)K7Br
A)PBr
B)KBr
C)KBr2
D)K2Br
E)K7Br

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
What is the formula for the ionic compound lithium oxide?
A)LiO
B)LiO2
C)Li2O
D)Li2O2
A)LiO
B)LiO2
C)Li2O
D)Li2O2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
What is the electron configuration of an F- ion?
A)1s22s22p4
B)1s22s22p5
C)1s22s22p6
D)1s22s22p63s1
A)1s22s22p4
B)1s22s22p5
C)1s22s22p6
D)1s22s22p63s1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
What is the ion symbol for an atom with twenty (20)protons and eighteen (18)electrons?
A)Ca
B)Ca2+
C)Ar
D)Ar2+
E)Ar2-
A)Ca
B)Ca2+
C)Ar
D)Ar2+
E)Ar2-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
When forming ions, metals typically do which of the following?
A)lose electrons to form negatively charged ions
B)gain protons to form cations
C)gain electrons to form anions
D)lose electrons to form positively charged ions
A)lose electrons to form negatively charged ions
B)gain protons to form cations
C)gain electrons to form anions
D)lose electrons to form positively charged ions

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
How many protons and electrons are present in Co2+?
A)27 protons, 27 electrons
B)27 protons, 29 electrons
C)27 protons, 25 electrons
D)29 protons, 27 electrons
A)27 protons, 27 electrons
B)27 protons, 29 electrons
C)27 protons, 25 electrons
D)29 protons, 27 electrons

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
What is the charge of the ions formed by group 7A elements?
A)+1
B)+7
C)-1
D)-7
A)+1
B)+7
C)-1
D)-7

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
What is the ion symbol for an atom with sixteen (16)protons and eighteen (18)electrons?
A)S2-
B)S
C)Ar
D)Ar2+
E)Ar2-
A)S2-
B)S
C)Ar
D)Ar2+
E)Ar2-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement concerning the ion Li+ is correct?
A)Li+ has two total electrons.
B)Li+ has an octet of electrons in its valence shell.
C)Li+ is formed when a lithium atom gains one electron.
D)Li+ is formed when a lithium atom gains one proton.
A)Li+ has two total electrons.
B)Li+ has an octet of electrons in its valence shell.
C)Li+ is formed when a lithium atom gains one electron.
D)Li+ is formed when a lithium atom gains one proton.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement best explains the basis for the octet rule?
A)Atoms are most stable when they have eight bonds.
B)Atoms lose, gain, or share electrons to become a stable, noble gas.
C)Atoms are most stable with eight protons in their nucleus.
D)Atoms are most stable with eight electrons in their valence shell and the electron configuration of a noble gas.
A)Atoms are most stable when they have eight bonds.
B)Atoms lose, gain, or share electrons to become a stable, noble gas.
C)Atoms are most stable with eight protons in their nucleus.
D)Atoms are most stable with eight electrons in their valence shell and the electron configuration of a noble gas.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which pair of elements will form an ionic compound?
A)sulfur and oxygen
B)copper and iron
C)iron and chlorine
D)bromine and oxygen
A)sulfur and oxygen
B)copper and iron
C)iron and chlorine
D)bromine and oxygen

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
If atoms with the electron-dot symbols shown below are combined, what is the formula of the ionic compound that is formed? 
A)XY
B)X2Y
C)XY2
D)X2Y2
E)X3Y2

A)XY
B)X2Y
C)XY2
D)X2Y2
E)X3Y2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Which atom fits the electron-dot symbol shown below? 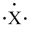
A)Li
B)B
C)N
D)Na
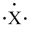
A)Li
B)B
C)N
D)Na

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
What is the formula for chromous cyanide?
A)CrCN
B)Cr(CN)2
C)Cr(CN)3
D)Cr2CN
A)CrCN
B)Cr(CN)2
C)Cr(CN)3
D)Cr2CN

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
What period 4 element forms an ion with a -1 charge?
A)sulfur
B)bromine
C)iodine
D)rubidium
E)potassium
A)sulfur
B)bromine
C)iodine
D)rubidium
E)potassium

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
What is the name of the SO42- ion?
A)sulfide ion
B)sulfate ion
C)sulfite ion
D)bisulfate ion
A)sulfide ion
B)sulfate ion
C)sulfite ion
D)bisulfate ion

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
What is the chemical formula for the ionic compound calcium selenide?
A)Ca2Se2
B)CaSe
C)CaSe2
D)Ca2Se
A)Ca2Se2
B)CaSe
C)CaSe2
D)Ca2Se

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
The correct name for Ag2O is _____.
A)silver (II)oxide
B)silver (I)oxide
C)silver oxide
D)disilver oxide
A)silver (II)oxide
B)silver (I)oxide
C)silver oxide
D)disilver oxide

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
What is the charge on the chromium ion in the ionic compound CrCl3?
A)+6
B)+3
C)-3
D)-6
E)Not enough information is given to determine the charge.
A)+6
B)+3
C)-3
D)-6
E)Not enough information is given to determine the charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
Which compound has the highest melting point?
A)KCl
B)CH4
C)C6H12O6
D)H2O
A)KCl
B)CH4
C)C6H12O6
D)H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
What is the chemical formula of tin (IV)cyanide?
A)Sn4CN
B)SnCN4
C)Sn(CN)4
D)SnC4N4
A)Sn4CN
B)SnCN4
C)Sn(CN)4
D)SnC4N4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
If atoms with the electron-dot symbols shown below are combined, what is the formula of the ionic compound that is formed? 
A)XY
B)X2Y
C)XY2
D)X2Y2
E)X3Y2

A)XY
B)X2Y
C)XY2
D)X2Y2
E)X3Y2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
An atom has the electron-dot symbol shown below. If this atom is a main group element, what charge will it form in its ionic state? 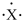
A)-1
B)+3
C)-5
D)-3
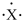
A)-1
B)+3
C)-5
D)-3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
What is the formula for the ionic compound formed from the lead (II)ion and the carbonate ion?
A)Pb(CO3)2
B)PbCO3
C)Pb2CO3
D)PbCO4
A)Pb(CO3)2
B)PbCO3
C)Pb2CO3
D)PbCO4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
Which chemical formula is correct?
A)Ag(CH3CO2)2
B)K2CN
C)Mg(OH)2
D)MgHCO3
E)More than one of the above formulas is correct.
A)Ag(CH3CO2)2
B)K2CN
C)Mg(OH)2
D)MgHCO3
E)More than one of the above formulas is correct.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
The correct name for AuI3 is
A)silver iodide
B)gold iodide
C)gold (III)iodide
D)gold iodide (III)
A)silver iodide
B)gold iodide
C)gold (III)iodide
D)gold iodide (III)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
What is the proper name for MgF2?
A)magnesium fluoride
B)magnesium (I)fluoride
C)magnesium (II)fluoride
D)magnesium difluoride
A)magnesium fluoride
B)magnesium (I)fluoride
C)magnesium (II)fluoride
D)magnesium difluoride

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
What is the proper name for CuCl?
A)copper chloride
B)copper (I)chloride
C)copper (II)chloride
D)copper monochloride
A)copper chloride
B)copper (I)chloride
C)copper (II)chloride
D)copper monochloride

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
The formula for zinc acetate is _____.
A)ZnCH3CO2
B)Zn(CH3CO2)2
C)Zn(HCO3)2
D)Zn(HCl)8
A)ZnCH3CO2
B)Zn(CH3CO2)2
C)Zn(HCO3)2
D)Zn(HCl)8

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Which chemical formula is not correct?
A)Na2HCO3
B)NaNO3
C)Na2HPO4
D)Na2SO3
E)More than one of the above formulas is not correct.
A)Na2HCO3
B)NaNO3
C)Na2HPO4
D)Na2SO3
E)More than one of the above formulas is not correct.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
What is the name of the compound (NH4)2SO4?
A)ammonium sulfide
B)ammonia sulfate
C)ammonia sulfoxide
D)ammonium sulfate
E)diammonium sulfate
A)ammonium sulfide
B)ammonia sulfate
C)ammonia sulfoxide
D)ammonium sulfate
E)diammonium sulfate

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
Ionic compounds are composed of positively and negatively charged ions held together by strong electrostatic forces.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
The name of an anion usually ends in the -ide if it is derived from a single atom or -ate (or -ite)if it is polyatomic.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
Ionic compounds have very high melting points compared to covalent compounds.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
A polyatomic ion is an ion that forms more than one charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Ionic bonds result from the sharing of electrons between two atoms.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Bonding is the joining of two atoms in a stable arrangement.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
The diphosphate ion is a biologically important polyatomic ion. If the ionic compound calcium diphosphate has the formula Ca2P2O7, which correctly represents the ion symbol of the diphosphate ion?
A)P2O72-
B)PO4-
C)2 PO46-
D)P2O74-
A)P2O72-
B)PO4-
C)2 PO46-
D)P2O74-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Neutral atoms always contain an equal number of protons, neutrons, and electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Elements in group 2A form ions with a +2 charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
The ionic formula for zinc oxide is ZnO2.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
The symbol for the nitrite ion is NO2-.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
All ionic compounds are soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
The correct name for CaHPO4 is calcium phosphate.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
The (II)in the name of the ionic compound lead (II)acetate specifically indicates that there are two lead ions present in the compound.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
The noble gas that has the same electronic configuration as the iodide ion is krypton.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Ionic compounds are composed of individual molecules, discrete groups of atoms that share electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
An ionic compound is a pure substance formed by chemically combining two or more nonmetal atoms together.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
Anions are formed when a neutral atom gains one or more electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
A cation is positively charged, and has more electrons than the neutral atom.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Ionic compounds are usually solids at room temperature.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
In crystal of an ionic compound, the cations are surrounded by anions so that the charges of the ions are balanced.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
An iodide ion has 53 protons and 54 electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
If the main group element has an electron-dot symbol of 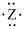 , the charge of the resulting ion will be -2.
, the charge of the resulting ion will be -2.
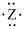 , the charge of the resulting ion will be -2.
, the charge of the resulting ion will be -2.
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
All isotopes of an element form the same type of ion.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
The formula for copper (II)hydroxide is Cu(OH)2.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
The electron configuration for the sulfide ion is 1s22s22p63s23p64s2.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
The carbonate ion contains the same number of oxygen atoms as the sulfate ion.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
The ion symbol for the ferric ion is Fe2+.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
The sulfite ion contains more oxygen atoms than the sulfate ion.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
An iron (III)ion has 29 protons and 26 electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
All p block elements form ions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
All polyatomic ions are anions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
The formula for stannic fluoride is SnF4.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
Strontium and barium form ions with the same charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Potassium sulfide has the chemical formula K2S.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Sodium nitrate and sodium nitrite are two acceptable names for the same compound.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
A main group element is especially stable when it possesses eight total electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
The chemical formulas of ionic compounds are written with the cation first, and then the anion, with subscripts to show how many of each are needed to have zero net charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Sodium bicarbonate and sodium hydrogen carbonate are two acceptable names for the same compound.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
If the main group element has an electron-dot symbol of 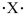 , the charge of the resulting ion will be +2.
, the charge of the resulting ion will be +2.
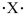 , the charge of the resulting ion will be +2.
, the charge of the resulting ion will be +2.
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


