Exam 3: Ionic Compounds
Exam 1: Matter and Measurement89 Questions
Exam 2: Atoms and the Periodic Table90 Questions
Exam 3: Ionic Compounds90 Questions
Exam 4: Covalent Compounds90 Questions
Exam 5: Chemical Reactions89 Questions
Exam 6: Energy Changes, Reaction Rates, and Equilibrium88 Questions
Exam 7: Gases, Liquids, and Solids79 Questions
Exam 8: Solutions90 Questions
Exam 9: Acids and Bases90 Questions
Exam 10: Nuclear Chemistry85 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups103 Questions
Exam 12: Alkanes106 Questions
Exam 13: Unsaturated Hydrocarbons101 Questions
Exam 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur111 Questions
Exam 15: The Three-Dimensional Shape of Molecules100 Questions
Exam 16: Aldehydes and Ketones103 Questions
Exam 17: Carboxylic Acids, Esters, and Amides81 Questions
Exam 18: Amines and Neurotransmitters105 Questions
Exam 19: Lipids105 Questions
Exam 20: Carbohydrates92 Questions
Exam 21: Amino Acids, Proteins, and Enzymes88 Questions
Exam 22: Nucleic Acids and Protein Synthesis89 Questions
Exam 23: Digestion and the Conversion of Food Into Energy92 Questions
Exam 24: Carbohydrate, Lipid, and Protein Metabolism91 Questions
Select questions type
What is the formula for the ionic compound formed when aluminum and sulfur combine?
(Multiple Choice)
4.7/5  (25)
(25)
The electron configuration for the sulfide ion is 1s22s22p63s23p64s2.
(True/False)
4.7/5  (32)
(32)
The (II)in the name of the ionic compound lead (II)acetate specifically indicates that there are two lead ions present in the compound.
(True/False)
4.7/5  (30)
(30)
What is the formula for the ionic compound formed from the lead (II)ion and the carbonate ion?
(Multiple Choice)
4.8/5  (26)
(26)
Ionic compounds are composed of individual molecules, discrete groups of atoms that share electrons.
(True/False)
4.9/5  (37)
(37)
Ionic bonds result from the sharing of electrons between two atoms.
(True/False)
4.8/5  (28)
(28)
An atom has the electron-dot symbol shown below. If this atom is a main group element, what charge will it form in its ionic state? 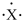
(Multiple Choice)
4.9/5  (33)
(33)
If the main group element has an electron-dot symbol of 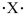 , the charge of the resulting ion will be +2.
, the charge of the resulting ion will be +2.
(True/False)
4.9/5  (36)
(36)
The name of an anion usually ends in the -ide if it is derived from a single atom or -ate (or -ite)if it is polyatomic.
(True/False)
4.8/5  (43)
(43)
What is the ion symbol for an atom with sixteen (16)protons and eighteen (18)electrons?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)