Deck 8: Applications of Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/171
Play
Full screen (f)
Deck 8: Applications of Aqueous Equilibria
1
Calculate the pH of a solution prepared by mixing 50 mL of a 0.10 M solution of HF with 25 mL of a 0.20 M solution of NaF. pKa of HF is 3.14.
A) 5.83
B) 12.00
C) 3.14
D) 7.35
E) 10.80
A) 5.83
B) 12.00
C) 3.14
D) 7.35
E) 10.80
3.14
2
A student uses 16.60 mL of 0.100 M NaOH to titrate a 0.2000-g sample of an unknown acid. Which of the following acids is the unknown most likely to be? Assume that only the hydrogens bonded to oxygen are titrated by the sodium hydroxide and that, because of experimental error, the student's value may not be identical to the theoretical value.
A) oxalic acid, HOOCCOOH, molar mass 90
B) succinic acid, HOOCCH2CH2COOH, molar mass 118
C) benzoic acid, C6H5COOH, molar mass 122
D) phthalic acid, C6H4 (COOH)2, molar mass 166
E) not enough information
A) oxalic acid, HOOCCOOH, molar mass 90
B) succinic acid, HOOCCH2CH2COOH, molar mass 118
C) benzoic acid, C6H5COOH, molar mass 122
D) phthalic acid, C6H4 (COOH)2, molar mass 166
E) not enough information
benzoic acid, C6H5COOH, molar mass 122
3
You have solutions of 0.200 M HNO2 and 0.200 M KNO2 (Ka for HNO2 = 4.00 *10-4). A buffer of pH 3.000 is needed. What volumes of HNO2 and KNO2 are required to make 1 L of buffered solution?
A) 286 mL HNO2; 714 mL KNO2
B) 714 mL HNO2; 286 mL KNO2
C) 413 mL HNO2; 587 mL KNO2
D) 500 mL of each
E) 587 mL HNO2; 413 mL KNO2
A) 286 mL HNO2; 714 mL KNO2
B) 714 mL HNO2; 286 mL KNO2
C) 413 mL HNO2; 587 mL KNO2
D) 500 mL of each
E) 587 mL HNO2; 413 mL KNO2
714 mL HNO2; 286 mL KNO2
4
What is the pH of a solution that results when 0.012 mol HNO3 is added to 670.0 mL of a solution that is 0.26 M in aqueous ammonia and 0.48 M in ammonium nitrate. Assume no volume change. (Kb for NH3 = 1.8*10-5.)
A) 8.94
B) 9.57
C) 5.06
D) 8.99
E) 4.43
A) 8.94
B) 9.57
C) 5.06
D) 8.99
E) 4.43

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
5
Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 *10-10). Which of the following statements is true?
A) The solution is not a buffer because [HCN] is not equal to [CN-].
B) [OH-] > [H+]
C) The pH will be below 7.00 because the concentration of the acid is greater than that of the base.
D) The buffer will be more resistant to pH changes from addition of strong acid than to pH changes from addition of strong base.
E) All of these statements are false.
A) The solution is not a buffer because [HCN] is not equal to [CN-].
B) [OH-] > [H+]
C) The pH will be below 7.00 because the concentration of the acid is greater than that of the base.
D) The buffer will be more resistant to pH changes from addition of strong acid than to pH changes from addition of strong base.
E) All of these statements are false.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
6
How many mmoles of HCl must be added to 130.0 mL of a 0.40 M solution of methylamine (pKb = 3.36) to give a buffer having a pH of 11.59?
A) 16 mmol
B) 0.11 mmol
C) 5.2 mmol
D) 2.5 mmol
E) 0.91 mmol
A) 16 mmol
B) 0.11 mmol
C) 5.2 mmol
D) 2.5 mmol
E) 0.91 mmol

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
7
Consider a solution consisting of the following two buffer systems: H2CO3 ![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B) [HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] C) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c155_d9e8_892c_37505a16b276_TB6420_11.jpg) HCO3- + H+ pKa = 6.4
HCO3- + H+ pKa = 6.4
H2PO4-![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B) [HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] C) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c155_d9e9_892c_97a858be7801_TB6420_11.jpg) HPO42- + H+ pKa = 7.2
HPO42- + H+ pKa = 7.2
At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
A) [H2CO3] > [HCO3-] and [H2PO4-] > [HPO42-]
B) [HCO3-] > [H2CO3] and [HPO42-] > [H2PO4-]
C) [H2CO3] > [HCO3-] and [HPO42-] > [H2PO4-]
D) [H2CO3] = [HCO3-] and [HPO42-] > [H2PO4-]
E) [H2CO3] = [HCO3-] and [H2PO4-] > [HPO42-]
![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B) [HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] C) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c155_d9e8_892c_37505a16b276_TB6420_11.jpg) HCO3- + H+ pKa = 6.4
HCO3- + H+ pKa = 6.4H2PO4-
![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B) [HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] C) [H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E) [H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c155_d9e9_892c_97a858be7801_TB6420_11.jpg) HPO42- + H+ pKa = 7.2
HPO42- + H+ pKa = 7.2At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
A) [H2CO3] > [HCO3-] and [H2PO4-] > [HPO42-]
B) [HCO3-] > [H2CO3] and [HPO42-] > [H2PO4-]
C) [H2CO3] > [HCO3-] and [HPO42-] > [H2PO4-]
D) [H2CO3] = [HCO3-] and [HPO42-] > [H2PO4-]
E) [H2CO3] = [HCO3-] and [H2PO4-] > [HPO42-]

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following will not produce a buffered solution?
A) 100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M HCl
B) 100 mL of 0.1 M NaHCO3 and 25 mL of 0.2 M HCl
C) 100 mL of 0.1 M Na2CO3 and 75 mL of 0.2 M HCl
D) 50 mL of 0.2 M Na2CO3 and 5 mL of 1.0 M HCl
E) 100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M NaOH
A) 100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M HCl
B) 100 mL of 0.1 M NaHCO3 and 25 mL of 0.2 M HCl
C) 100 mL of 0.1 M Na2CO3 and 75 mL of 0.2 M HCl
D) 50 mL of 0.2 M Na2CO3 and 5 mL of 1.0 M HCl
E) 100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M NaOH

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the pH of a solution that contains 3.25 M HCN (Ka = 6.2*10-10), 1.00 M NaOH and 1.50 M NaCN.
A) 8.28
B) 7.46
C) 9.25
D) 8.86
E) none of these
A) 8.28
B) 7.46
C) 9.25
D) 8.86
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate [H+] in a solution that is 0.24 M in NaF and 0.46 M in HF. (Ka = 7.2*10-4)
A) 1.1 * 10-4 M
B) 1.4 *10-3 M
C) 1.8 * 10-2 M
D) 3.8 * 10-4 M
E) 0.46 M
A) 1.1 * 10-4 M
B) 1.4 *10-3 M
C) 1.8 * 10-2 M
D) 3.8 * 10-4 M
E) 0.46 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
11
For 110.0 mL of a buffer that is 0.40 M in HOCl and 0.52 M in NaOCl, what is the pH after 11.2 mL of 1.1 M NaOH is added? Ka for HOCl = 3.5 *10-8. (Assume the volumes are additive.)
A) 7.36
B) 7.57
C) 7.34
D) 7.11
E) 7.80
A) 7.36
B) 7.57
C) 7.34
D) 7.11
E) 7.80

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the pH of a solution made by mixing 49.5 mL of 0.335 M NaA (Ka for HA = 1.0 * 10-9) with 30.6 mL of 0.120 M HCl.
A) 9.00
B) 9.65
C) 8.45
D) 8.75
E) 9.55
A) 9.00
B) 9.65
C) 8.45
D) 8.75
E) 9.55

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
13
Buffers in the human body
A) precipitate proteins so enzymes are inactive.
B) help to keep the body temperature constant.
C) help change the blood plasma pH when foods are eaten.
D) help to maintain a constant blood pH.
E) none of these
A) precipitate proteins so enzymes are inactive.
B) help to keep the body temperature constant.
C) help change the blood plasma pH when foods are eaten.
D) help to maintain a constant blood pH.
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
14
11.8 mL of 0.30 M HCl is added to a 122.0-mL sample of 0.210 M HNO2 (Ka for HNO2 = 4.0*10-4). What is the equilibrium concentration of NO2- ions?
A) 2.9 * 10-3 M
B) 2.6 * 10-2 M
C) 4.2*10-3 M
D) 5.5 * 10-4 M
E) 9.2 * 10-3 M
A) 2.9 * 10-3 M
B) 2.6 * 10-2 M
C) 4.2*10-3 M
D) 5.5 * 10-4 M
E) 9.2 * 10-3 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
15
A solution contains 0.34 M HA (Ka = 2.0 *10-7) and 0.17 M NaA. Calculate the pH after 0.05 mol of NaOH is added to 1.00 L of this solution.
A) 6.58
B) 6.70
C) 6.40
D) 6.82
E) 6.19
A) 6.58
B) 6.70
C) 6.40
D) 6.82
E) 6.19

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
16
What combination of substances will give a buffered solution that has a pH of 5.05? Assume each pair of substances is dissolved in 5.0 L of water. (Kb for NH3 = 1.8 *10-5; Kb for C5H5N = 1.7 * 10-9)
A) 1.0 mol C5H5N and 1.5 mol C5H5NHCl
B) 1.0 mol NH3 and 1.5 mol NH4Cl
C) 1.5 mol NH3 and 1.0 mol NH4Cl
D) 1.5 mol C5H5N and 1.0 mol C5H5NHCl
E) none of these
A) 1.0 mol C5H5N and 1.5 mol C5H5NHCl
B) 1.0 mol NH3 and 1.5 mol NH4Cl
C) 1.5 mol NH3 and 1.0 mol NH4Cl
D) 1.5 mol C5H5N and 1.0 mol C5H5NHCl
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
17
How much solid NaCN must be added to 1.0 L of a 0.5 M HCN solution to produce a solution with pH 7.0? Ka = 6.2 *10-10 for HCN.
A) 0.0034 g
B) 0.15 g
C) 160 g
D) 24 g
E) 11 g
A) 0.0034 g
B) 0.15 g
C) 160 g
D) 24 g
E) 11 g

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
18
How many moles of HCl need to be added to 150.0 mL of 0.50 M NaZ to have a solution with a pH of 6.50? (Ka of HZ is 2.3* 10-5.) Assume negligible volume of the HCl.
A) 6.8 * 10-3 mol
B) 7.5 *10-2 mol
C) 1.0 *10-3 mol
D) 5.0 * 10-1 mol
E) none of these
A) 6.8 * 10-3 mol
B) 7.5 *10-2 mol
C) 1.0 *10-3 mol
D) 5.0 * 10-1 mol
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
19
You are given a solution of the weak base Novocain, Nvc. Its pH is 11.00. You add to the solution a small amount of a salt containing the conjugate acid of Novocain, NvcH+. Which statement is true?
A) The pH and the pOH remain unchanged.
B) The pH and the pOH both decrease.
C) The pH and the pOH both increase.
D) The pH increases and pOH decreases.
E) The pH decreases and the pOH increases.
A) The pH and the pOH remain unchanged.
B) The pH and the pOH both decrease.
C) The pH and the pOH both increase.
D) The pH increases and pOH decreases.
E) The pH decreases and the pOH increases.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
20
For ammonia, Kb is 1.8 *10-5 . To make a buffered solution with pH 10.0, the ratio of NH4Cl to NH3 must be
A) 1.8 : 1.
B) 0.18 : 1.
C) 1 : 1.8.
D) 1 : 0.18.
E) none of these
A) 1.8 : 1.
B) 0.18 : 1.
C) 1 : 1.8.
D) 1 : 0.18.
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
21
One milliliter (1.00 mL) of acid taken from a lead storage battery is pipetted into a flask. Water and phenolphthalein indicator are added, and the solution is titrated with 0.44 M NaOH until a pink color appears; 13.6 mL is required. Find, to within 5%, the number of grams of H2SO4 (formula weight = 98) present in 1 L of the battery acid.
A) 480 g
B) 240 g
C) 580 g
D) 290 g
E) 750 g
A) 480 g
B) 240 g
C) 580 g
D) 290 g
E) 750 g

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
22
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 *10-2, Ka2 = 2.03 * 10-6.
-Calculate [H+] after 300.0 mL of 1.00 M NaOH has been added.
A) 1.00 * 10-7 M
B) 4.00 * 10-14 M
C) 1.41 * 10-10 M
D) 1.00 M
E) none of these
-Calculate [H+] after 300.0 mL of 1.00 M NaOH has been added.
A) 1.00 * 10-7 M
B) 4.00 * 10-14 M
C) 1.41 * 10-10 M
D) 1.00 M
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
23
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 *10-2, Ka2 = 2.03 * 10-6.
-Calculate [H+] after 150.0 mL of 1.00 M NaOH has been added.
A) 2.03 *10-6 M
B) 1.41 * 10-10 M
C) 1.49 *10-2 M
D) 1.00 * 10-7 M
E) none of these
-Calculate [H+] after 150.0 mL of 1.00 M NaOH has been added.
A) 2.03 *10-6 M
B) 1.41 * 10-10 M
C) 1.49 *10-2 M
D) 1.00 * 10-7 M
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
24
A 50.00-mL sample of 0.100 M KOH is titrated with 0.100 M HNO3. Calculate the pH of the solution after the 52.00 mL of HNO3 is added.
A) 6.50
B) 3.01
C) 2.41
D) 2.71
E) none of these
A) 6.50
B) 3.01
C) 2.41
D) 2.71
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
25
If 22 mL of 0.50 M HCl is added to 112 mL of 0.20 M NaOH, what is the final pH?
A) 0.78
B) 13.22
C) 12.93
D) 7.00
E) 1.07
A) 0.78
B) 13.22
C) 12.93
D) 7.00
E) 1.07

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
26
A 65.5-mL sample of 0.14 M HNO2 (Ka = 4.0 * 10-4) is titrated with 0.11 M NaOH. What is the pH after 26.8 mL of NaOH has been added?
A) 10.28
B) 7.00
C) 3.72
D) 2.91
E) 3.07
A) 10.28
B) 7.00
C) 3.72
D) 2.91
E) 3.07

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
27
A solution of hydrochloric acid of unknown concentration was titrated with 0.11 M NaOH. If a 150-mL sample of the HCl solution required exactly 12 mL of the NaOH solution to reach the equivalence point, what was the pH of the HCl solution?
A) 11.94
B) 2.09
C) 0.11
D) 2.06
E) -0.14
A) 11.94
B) 2.09
C) 0.11
D) 2.06
E) -0.14

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of HCl(g) must be added to 1.0 L of 2.0 M NaOH to achieve a pH of 0.00? (Neglect any volume change.)
A) 3.0 mol
B)10. mol
C) 1.0 mol
D) 2.0 mol
E) none of these
A) 3.0 mol
B)10. mol
C) 1.0 mol
D) 2.0 mol
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
29
Equal volumes of 0.1 M HCl and 0.1 M HC2H3O2 are titrated with 0.1 M NaOH. Which of the following would be equal for both titrations?
A) the pH at the equivalence point
B) the volume of NaOH added to reach the equivalence point
C) the initial pH
D) the pH at the halfway point
E) two of the above
A) the pH at the equivalence point
B) the volume of NaOH added to reach the equivalence point
C) the initial pH
D) the pH at the halfway point
E) two of the above

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
30
A 10-mL sample of tartaric acid is titrated to a phenolphthalein endpoint with 20. mL of 1.0 M NaOH. Assuming tartaric acid is diprotic, what is the molarity of the acid?
A)10.
B) 1.0
C) 2.0
D) 4.0
E) impossible to determine
A)10.
B) 1.0
C) 2.0
D) 4.0
E) impossible to determine

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
31
You are given 5.00 mL of an H2SO4 solution of unknown concentration. You divide the 5.00-mL sample into five 1.00-mL samples and titrate each separately with 0.1000 M NaOH. In each titration, the H2SO4 is completely neutralized. The average volume of NaOH solution used to reach the endpoint is 15.3 mL. What was the concentration of H2SO4 in the 5.00-mL sample?
A) 0.306 M
B) 0.765 M
C) 3.06 M
D) 1.53 * 10-3 M
E) 1.53 M
A) 0.306 M
B) 0.765 M
C) 3.06 M
D) 1.53 * 10-3 M
E) 1.53 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
32
What is the molarity of a sodium hydroxide solution if 25.0 mL of this solution reacts exactly with 22.30 mL of 0.253 M sulfuric acid?
A) 0.284 M
B) 0.451 M
C) 0.567 M
D) 0.226 M
E) 0.113 M
A) 0.284 M
B) 0.451 M
C) 0.567 M
D) 0.226 M
E) 0.113 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
33
What quantity of NaOH(s) must be added to 1.00 L of 0.200 M HCl to achieve a pH of 12.00? (Assume no volume change.)
A) 0.200 mol
B) 0.010 mol
C) 0.210 mol
D) 0.420 mol
E) none of these
A) 0.200 mol
B) 0.010 mol
C) 0.210 mol
D) 0.420 mol
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following solutions will be the best buffer at a pH of 9.26? (Ka for HC2H3O2 is 1.8 * 10-5; Kb for NH3 is 1.8 *10-5.)
A) 0.20 M NH3 and 0.20 M NH4Cl
B) 3.0 M HC2H3O2 and 3.0 M NH4Cl
C) 0.20 M HC2H3O2 and 0.20 M NaC2H3O2
D) 3.0 M NH3 and 3.0 M NH4Cl
E) 3.0 M HC2H3O2 and 3.0 M NH3
A) 0.20 M NH3 and 0.20 M NH4Cl
B) 3.0 M HC2H3O2 and 3.0 M NH4Cl
C) 0.20 M HC2H3O2 and 0.20 M NaC2H3O2
D) 3.0 M NH3 and 3.0 M NH4Cl
E) 3.0 M HC2H3O2 and 3.0 M NH3

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
35
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 *10-2, Ka2 = 2.03 * 10-6.
-Calculate the [H+] after 50.00 mL of 1.00 M NaOH has been added.
A) 2.00 * 10-6 M
B) 1.41 *10-10 M
C) 1.49 *10-2 M
D) 3.87 * 10-2 M
E) none of these
-Calculate the [H+] after 50.00 mL of 1.00 M NaOH has been added.
A) 2.00 * 10-6 M
B) 1.41 *10-10 M
C) 1.49 *10-2 M
D) 3.87 * 10-2 M
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
36
A 75.0-mL sample of 0.0500 M HCN (Ka = 6.2 *10-10) is titrated with 0.500 M NaOH. What is [H+] in the solution after 3.0 mL of 0.500 M NaOH has been added?
A) 9.3 *10-10 M
B) 5.2 * 10-13 M
C) 1.0 * 10-7 M
D) 4.1 * 10-10 M
E) none of these
A) 9.3 *10-10 M
B) 5.2 * 10-13 M
C) 1.0 * 10-7 M
D) 4.1 * 10-10 M
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
37
The pH at the equivalence point of a titration of a weak acid with a strong base is
A) More data are needed to answer this question.
B) equal to 7.00.
C) greater than 7.00.
D) less than 7.00.
A) More data are needed to answer this question.
B) equal to 7.00.
C) greater than 7.00.
D) less than 7.00.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
38
What volume of 0.0100 M NaOH must be added to 1.00 L of 0.0500 M HOCl to achieve a pH of 8.00? Ka for HOCl is 3.5*10-8.
A) 1.2 L
B) 1.0 L
C) 3.9 L
D) 5.0 L
E) none of these
A) 1.2 L
B) 1.0 L
C) 3.9 L
D) 5.0 L
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the net ionic equation for the reaction that occurs during the titration of nitrous acid with potassium hydroxide?
A) HNO2 + KOH K+ + NO2- + H2O
B) H+ + OH- H2O
C) HNO2 + OH- NO2- + H2O
D) HNO2 + H2O NO2- + H3O+
E) HNO2 + K+ + OH- KNO2 + H2O
A) HNO2 + KOH K+ + NO2- + H2O
B) H+ + OH- H2O
C) HNO2 + OH- NO2- + H2O
D) HNO2 + H2O NO2- + H3O+
E) HNO2 + K+ + OH- KNO2 + H2O

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
40
A 59.00-mL sample of 0.0650 M HCN (Ka = 6.2 * 10-10) is titrated with 0.670 M NaOH. What volume of 0.670 M NaOH is required to reach the stoichiometric point?
A) 5.72 mL
B) 59.0 mL
C) 608 mL
D) 0.00164 mL
E) 3.84 mL
A) 5.72 mL
B) 59.0 mL
C) 608 mL
D) 0.00164 mL
E) 3.84 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
41
In the titration of a weak acid HA with 0.100 M NaOH, the stoichiometric point is known to occur at a pH value of approximately 10. Which of the following indicator acids would be best to use to mark the endpoint of this titration?
A) indicator A, Ka = 10-14
B) indicator C, Ka = 10-8
C) indicator D, Ka = 10-6
D) indicator B, Ka = 10-11
E) none of these
A) indicator A, Ka = 10-14
B) indicator C, Ka = 10-8
C) indicator D, Ka = 10-6
D) indicator B, Ka = 10-11
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
42
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 350.0 mL of 0.200 M NaOH is added
A) A3-
B) HA2-, A3-
C) HA2-
D) OH-, A3-
E) H2A-, HA2-
After 350.0 mL of 0.200 M NaOH is added
A) A3-
B) HA2-, A3-
C) HA2-
D) OH-, A3-
E) H2A-, HA2-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 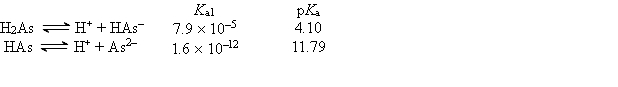 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 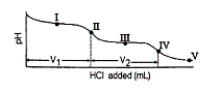
Which of the following is a major species present at point IV?
A) As2-
B) H+
C) H2As
D) HAs-
E) none of these
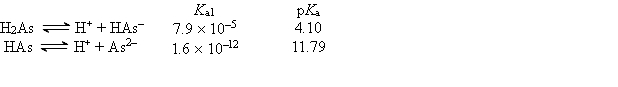 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
Which of the following is a major species present at point IV?
A) As2-
B) H+
C) H2As
D) HAs-
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
44
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 200.0 mL of 0.200 M NaOH is added
A) HA2-, A3-
B) HA2-
C) H2A-
D) H2A-, HA2-
E) A3-
After 200.0 mL of 0.200 M NaOH is added
A) HA2-, A3-
B) HA2-
C) H2A-
D) H2A-, HA2-
E) A3-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
45
A certain indicator HIn has a pKa of 9.00, and a color change becomes visible when 7.00% of it is In-. At what pH is this color change visible?
A) 6.15
B) 7.88
C) 10.2
D) 3.85
E) none of these
A) 6.15
B) 7.88
C) 10.2
D) 3.85
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
46
After adding 25.0 mL of 0.100 M NaOH to 100.0 mL of 0.100 M weak acid (HA), the pH is found to be 5.90. Determine the value of Ka for the acid HA.
A) 4.2 * 10-7
B) 3.5 * 10-9
C) 1.6 *10-11
D) 2.1 * 10-5
E) none of these
A) 4.2 * 10-7
B) 3.5 * 10-9
C) 1.6 *10-11
D) 2.1 * 10-5
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 *10-10) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)
A)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_46ba_892c_d9b9c8fe117e_TB6420_11.jpg)
B)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_46bb_892c_e344a40965fe_TB6420_11.jpg)
C)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_6dcc_892c_3fd0d84a473f_TB6420_11.jpg)
D) [H+] = x
E) none of these
A)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_46ba_892c_d9b9c8fe117e_TB6420_11.jpg)
B)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_46bb_892c_e344a40965fe_TB6420_11.jpg)
C)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 *10<sup>-</sup><sup>10</sup>) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)</strong> A) B) C) D) [H<sup>+</sup>] = x E) none of these](https://storage.examlex.com/TB6420/11eaaf8d_c15a_6dcc_892c_3fd0d84a473f_TB6420_11.jpg)
D) [H+] = x
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
48
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH. The student then adds 13.0 mL of 0.100 M HCl. The pH of the resulting solution is 4.7. Which of the following statements is true?
A) At pH 4.7, half of the conjugate base, A-, has been converted to HA.
B) The pKa of the acid is 4.7.
C) The pKa of the acid is less than 4.7.
D) The pKa of the acid is greater than 4.7.
E) More than one of the above statements are correct.
A) At pH 4.7, half of the conjugate base, A-, has been converted to HA.
B) The pKa of the acid is 4.7.
C) The pKa of the acid is less than 4.7.
D) The pKa of the acid is greater than 4.7.
E) More than one of the above statements are correct.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
49
A 100.0-mL sample of 0.2 M (CH3)3N (Kb = 5.3 * 10-5) is titrated with 0.2 M HCl. What is the pH at the equivalence point?
A) 5.4
B) 7.0
C) 10.3
D) 3.1
E) 9.9
A) 5.4
B) 7.0
C) 10.3
D) 3.1
E) 9.9

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the pH at the equivalence point for the titration of 1.0 M ethylamine, C2H5NH2, by 1.0 M perchloric acid, HClO4. (pKb for C2H5NH2 = 3.25)
A) 6.05
B) 2.24
C) 2.09
D) 5.38
E) 5.53
A) 6.05
B) 2.24
C) 2.09
D) 5.38
E) 5.53

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 *10-6, Ka2 = 1.0 *10-10) is titrated with the following volumes of 1.00 M NaOH. 100.0 mL of 1.00 M NaOH
A) 3.35
B) 8.00
C) 3.00
D) 6.00
E) none of these
A) 3.35
B) 8.00
C) 3.00
D) 6.00
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
52
An indicator HIn has Ka = 1 *10-8. At pH = 6.0, what is the ratio HIn/In-?
A) 1/100
B) 10/1
C) 1/1
D) 100/1
E) none of these
A) 1/100
B) 10/1
C) 1/1
D) 100/1
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
53
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 0 mL of 0.200 M NaOH is added
A) H3A, H2A-, HA2-, A3-
B) H3A, H2A-
C) H3A
D) H2A-, HA2-
E) H2A-
After 0 mL of 0.200 M NaOH is added
A) H3A, H2A-, HA2-, A3-
B) H3A, H2A-
C) H3A
D) H2A-, HA2-
E) H2A-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 *10-10) with 0.500 M HCl. Calculate the pH of the solution at the stoichiometric point.
A) 8.70
B) 2.68
C) 11.62
D) -0.85
E) none of these
A) 8.70
B) 2.68
C) 11.62
D) -0.85
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
55
A 100.-mL sample of 0.10 M HCl is mixed with 50. mL of 0.10 M NH3. What is the resulting pH? (Kb for NH3 = 1.8 *10-5)
A) 7.85
B) 1.30
C) 3.87
D) 1.48
E) 12.52
A) 7.85
B) 1.30
C) 3.87
D) 1.48
E) 12.52

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
56
44.4 mL of a 1.42 M NaOH solution is titrated with a 1.90 M HCl solution. What is the final volume of the solution when the NaOH has been completely neutralized by the HCl? (Assume the volumes are additive.)
A) 33.2 mL
B) 77.6 mL
C) 59.4 mL
D) 45.1 mL
E) 104 mL
A) 33.2 mL
B) 77.6 mL
C) 59.4 mL
D) 45.1 mL
E) 104 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
57
A solution containing 10. mmol of CO32- and 5.0 mmol of HCO3- is titrated with 1.0 M HCl.
What volume of HCl must be added to reach the first equivalence point?
A) 5.0 mL
B) 15 mL
C)10. Ml
D) 25 mL
E)20. mL
What volume of HCl must be added to reach the first equivalence point?
A) 5.0 mL
B) 15 mL
C)10. Ml
D) 25 mL
E)20. mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
58
In the titration of a weak acid HA with 0.100 M NaOH, the stoichiometric point is known to occur at a pH value of approximately 11. Which of the following indicators would be best to use to mark the endpoint of this titration?
A) an indicator with Ka = 10-11
B) an indicator with Ka = 10-14
C) an indicator with Ka = 10-10
D) an indicator with Ka = 10-8
E) an indicator with Ka = 10-12
A) an indicator with Ka = 10-11
B) an indicator with Ka = 10-14
C) an indicator with Ka = 10-10
D) an indicator with Ka = 10-8
E) an indicator with Ka = 10-12

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
59
Methyl orange is an indicator with a Ka of 1 * 10-4. Its acid form, HIn, is red, while its base form, In-, is yellow. At pH 6.0, the indicator will be
A) yellow.
B) orange.
C) red.
D) blue.
E) not enough information
A) yellow.
B) orange.
C) red.
D) blue.
E) not enough information

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 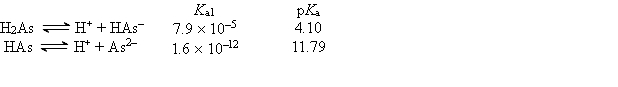 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
What major species is(are) present at point III?
A) HAs- only
B) H2As and H+
C) H2As only
D) As2- and HAs-
E) HAs- and H2As
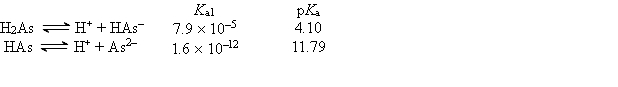 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
What major species is(are) present at point III?
A) HAs- only
B) H2As and H+
C) H2As only
D) As2- and HAs-
E) HAs- and H2As

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
61
A solution containing 10. mmol of CO32- and 5.0 mmol of HCO3- is titrated with 1.0 M HCl.
What total volume of HCl must be added to reach the second equivalence point?
A)20. mL
B) 5.0 mL
C)30. mL
D)10. mL
E) 25 mL
What total volume of HCl must be added to reach the second equivalence point?
A)20. mL
B) 5.0 mL
C)30. mL
D)10. mL
E) 25 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
62
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 220.0 mL of 0.200 M NaOH is added
A) A3-
B) OH-, A3-
C) H2A-, HA2-
D) HA2-
E) HA2-, A3-
After 220.0 mL of 0.200 M NaOH is added
A) A3-
B) OH-, A3-
C) H2A-, HA2-
D) HA2-
E) HA2-, A3-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
63
A 0.210-g sample of an acid (molar mass = 192 g/mol) is titrated with 30.5 mL of 0.108 M NaOH to a phenolphthalein endpoint. The formula of the acid is
A) H3A.
B) H2A.
C) H4A.
D) HA.
E) not enough information given
A) H3A.
B) H2A.
C) H4A.
D) HA.
E) not enough information given

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
64
A solution contains 10. mmol of H3PO4 and 5.0 mmol of NaH2PO4. How many milliliters of 0.10 M NaOH must be added to reach the second equivalence point of the titration of the H3PO4 with NaOH?
A) 2.0 * 102 mL
B) 150 mL
C) 250 mL
D) 1.0 *102 mL
E) 50 mL
A) 2.0 * 102 mL
B) 150 mL
C) 250 mL
D) 1.0 *102 mL
E) 50 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
65
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 * 10-6, Ka2 = 1.0 * 10-10) is titrated with the following volumes of 1.00 M NaOH.
-150.0 mL of 1.00 M NaOH
A) 6.00
B) 6.48
C) 8.00
D) 8.50
E) None of these
-150.0 mL of 1.00 M NaOH
A) 6.00
B) 6.48
C) 8.00
D) 8.50
E) None of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
66
A 5.95-g sample of an acid, H2X, requires 45.0 mL of a 0.500 M NaOH solution for complete reaction (removing both protons). What is the molar mass of the acid expressed in grams per mol?
A) 529
B) 264
C) 178
D) 132
E) none of these
A) 529
B) 264
C) 178
D) 132
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
67
A 135.0-mL sample of a 0.25 M solution of H3PO4 is titrated with 0.12 M NaOH. What volume of base must be added to reach the third equivalence point?
A) 5.6 *102 mL
B) 2.8* 102 mL
C) 8.4 *102 mL
D) 1.9 * 102 mL
E) 94 mL
A) 5.6 *102 mL
B) 2.8* 102 mL
C) 8.4 *102 mL
D) 1.9 * 102 mL
E) 94 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
68
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 75.0 mL of 0.200 M NaOH is added
A) H2A-
B) HA2-
C) H3A, H2A-
D) H2A-, HA2-
E) H3A
After 75.0 mL of 0.200 M NaOH is added
A) H2A-
B) HA2-
C) H3A, H2A-
D) H2A-, HA2-
E) H3A

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 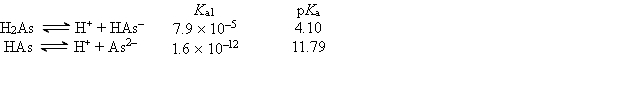 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 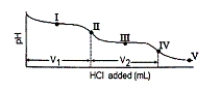
What is the pH at point I (V1/2 HCl added)?
A) 7.95
B) 12.39
C) 10
D) 11.79
E) none of these
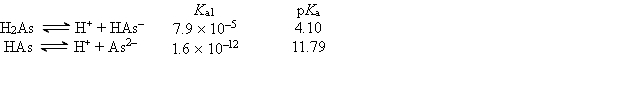 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
What is the pH at point I (V1/2 HCl added)?
A) 7.95
B) 12.39
C) 10
D) 11.79
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
70
For carbonic acid (H2CO3), Ka1 = 4.30 *10-7 and Ka2 = 5.62 *10-11. Calculate the pH of a 0.50 M solution of Na2CO3.
A) 11.97
B) 8.31
C) 10.67
D) 2.03
E) 3.33
A) 11.97
B) 8.31
C) 10.67
D) 2.03
E) 3.33

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
71
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 * 10-6, Ka2 = 1.0 * 10-10) is titrated with the following volumes of 1.00 M NaOH.
-0 mL of 1.00 M NaOH
A) 3.00
B) 6.00
C) 0
D) 3.35
E) None of these
-0 mL of 1.00 M NaOH
A) 3.00
B) 6.00
C) 0
D) 3.35
E) None of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
72
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 * 10-6, Ka2 = 1.0 * 10-10) is titrated with the following volumes of 1.00 M NaOH.
-200.0 mL of 1.00 M NaOH
A) 9.50
B) 6.48
C) 6.00
D) 8.00
E) None of these
-200.0 mL of 1.00 M NaOH
A) 9.50
B) 6.48
C) 6.00
D) 8.00
E) None of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
73
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 300.0 mL of 0.200 M NaOH is added
A) A3-
B) OH-, A3-
C) H3A, H2A-, HA2-, A3-
D) H2A-, OH-
E) OH-, HA2-, A3-
After 300.0 mL of 0.200 M NaOH is added
A) A3-
B) OH-, A3-
C) H3A, H2A-, HA2-, A3-
D) H2A-, OH-
E) OH-, HA2-, A3-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
74
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
After 100.0 mL of 0.200 M NaOH is added
A) H3A, HA2-, A3-
B) H2A-, HA2-
C) H3A, H2A-
D) H2A-
E) HA2-
After 100.0 mL of 0.200 M NaOH is added
A) H3A, HA2-, A3-
B) H2A-, HA2-
C) H3A, H2A-
D) H2A-
E) HA2-

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
75
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 * 10-6, Ka2 = 1.0 * 10-10) is titrated with the following volumes of 1.00 M NaOH.
-250.0 mL of 1.00 M NaOH
A) 10.00
B) 9.52
C) 8.00
D) 9.00
E) None of these
-250.0 mL of 1.00 M NaOH
A) 10.00
B) 9.52
C) 8.00
D) 9.00
E) None of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
76
You dissolve 1.24 g of an unknown diprotic acid in 220.0 mL of H2O. This solution is just neutralized by 6.44 mL of a 1.25 M NaOH solution. What is the molar mass of the unknown acid?
A) 1.28 * 102 g/mol
B) 3.08 * 102 g/mol
C) 2.10 * 102 g/mol
D) 1.54 * 102 g/mol
E) 1.93 *102 g/mol
A) 1.28 * 102 g/mol
B) 3.08 * 102 g/mol
C) 2.10 * 102 g/mol
D) 1.54 * 102 g/mol
E) 1.93 *102 g/mol

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 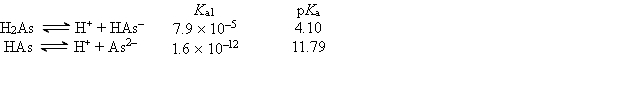 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
What is the pH at point III?
A) 7.95
B) 11.79
C) 12.39
D) 4.10
E) none of these
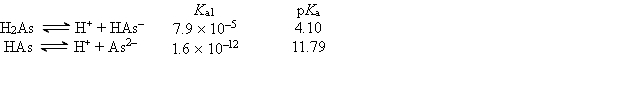 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 
What is the pH at point III?
A) 7.95
B) 11.79
C) 12.39
D) 4.10
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the titration of 200.0 mL of a 0.100 M solution of the weak acid H2A with 0.200 M NaOH. The first equivalence point is reached after 100.0 mL of 0.200 M NaOH has been added, and the pH is 6.27. The pH after 65.0 mL of 0.200 M NaOH has been added, the pH is 4.95. Calculate the pH of the original acid (no NaOH added).
A) 3.19
B) 2.84
C) 2.55
D) 4.68
E) none of these
A) 3.19
B) 2.84
C) 2.55
D) 4.68
E) none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
79
A solution contains 25 mmol of H3PO4 and 10. mmol of NaH2PO4. What volume of 2.0 M NaOH must be added to reach the second equivalence point of the titration of the H3PO4 with NaOH?
A)60. mL
B)30. mL
C) 5.0 mL
D) 12 mL
E) 25 mL
A)60. mL
B)30. mL
C) 5.0 mL
D) 12 mL
E) 25 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
80
In the titration of 100.0 mL of a 0.200 M solution of H2A (Ka1 = 1.0 * 10-5, Ka2 = 1.0 * 10-8), what volume of 0.400 M NaOH must be added to reach a pH of 5.00?
A) 25.0 mL
B) 100.0 mL
C) 0 mL
D) 150.0 mL
E) 50.0 mL
A) 25.0 mL
B) 100.0 mL
C) 0 mL
D) 150.0 mL
E) 50.0 mL

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck


