Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 10-3 M CuNO3. Cu(I) forms complex ions with cyanide as follows: ![A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 <font face=symbol></font> 10<sup>-3</sup> M CuNO<sub>3</sub>. Cu(I) forms complex ions with cyanide as follows: Calculate the following concentrations at equilibrium: -[Cu<sup>+</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c168_297e_892c_296022b8a32c_TB6420_00_TB6420_00_TB6420_00.jpg) Calculate the following concentrations at equilibrium:
-[Cu+]
Calculate the following concentrations at equilibrium:
-[Cu+]
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
A 0.210-g sample of an acid (molar mass = 192 g/mol) is titrated with 30.5 mL of 0.108 M NaOH to a phenolphthalein endpoint. The formula of the acid is
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
A
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 10-10) with 0.500 M HCl. Calculate the pH of the solution at the stoichiometric point.
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
B
A 100.0-mL sample of 0.2 M (CH3)3N (Kb = 5.3 10-5) is titrated with 0.2 M HCl. What is the pH at the equivalence point?
(Multiple Choice)
4.8/5  (36)
(36)
The value of Kf for the complex ion Ag(NH3)2+ is 1.7 107. Ksp for AgCl is 1.6 10-10. Calculate the molar solubility of AgCl in 1.0 M NH3.
(Multiple Choice)
4.8/5  (34)
(34)
A 75.0-mL sample of 0.0500 M HCN (Ka = 6.2 10-10) is titrated with 0.500 M NaOH. What is [H+] in the solution after 3.0 mL of 0.500 M NaOH has been added?
(Multiple Choice)
4.9/5  (40)
(40)
Derive the equation describing the relationship between the pH at the first equivalence point and the Ka values of the diprotic weak acid being titrated with NaOH.
(Essay)
4.9/5  (34)
(34)
Calculate the concentration of Ag+ in a saturated aqueous solution of Ag2CrO4. Ksp for Ag2CrO4 is 9.0 10-12.
(Multiple Choice)
4.7/5  (37)
(37)
A 59.00-mL sample of 0.0650 M HCN (Ka = 6.2 10-10) is titrated with 0.670 M NaOH. What volume of 0.670 M NaOH is required to reach the stoichiometric point?
(Multiple Choice)
4.9/5  (43)
(43)
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3)2+: 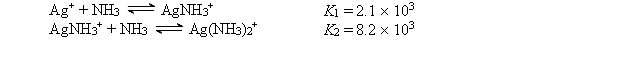 The concentration of Ag(NH3)2+ at equilibrium is
The concentration of Ag(NH3)2+ at equilibrium is
(Multiple Choice)
4.8/5  (39)
(39)
A solution contains 10. mmol of H3PO4 and 5.0 mmol of NaH2PO4. How many milliliters of 0.10 M NaOH must be added to reach the second equivalence point of the titration of the H3PO4 with NaOH?
(Multiple Choice)
4.9/5  (32)
(32)
The solubility of M(OH)2 in 0.010 M KOH is 1.0 10-5 mol/L. What is Ksp for M(OH)2?
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the pH of a solution that contains 3.25 M HCN (Ka = 6.2 10-10), 1.00 M NaOH and 1.50 M NaCN.
(Multiple Choice)
4.9/5  (44)
(44)
The solubility of Cd(OH)2 in water is 1.7 10-5 mol/L at 25°C. What is Ksp for Cd(OH)2?
(Multiple Choice)
4.8/5  (42)
(42)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 75.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.9/5  (42)
(42)
A solution containing 10. mmol of CO32- and 5.0 mmol of HCO3- is titrated with 1.0 M HCl.
What volume of HCl must be added to reach the first equivalence point?
(Multiple Choice)
4.7/5  (40)
(40)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-600.0 mL of 1.00 M NaOH
(Multiple Choice)
4.9/5  (34)
(34)
Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 10-10). Which of the following statements is true?
(Multiple Choice)
4.8/5  (39)
(39)
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 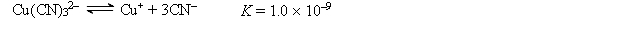 Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
Calculate the solubility of CuBr(s) (Ksp = 1.0 10-5) in 1.0 L of 1.0 M NaCN.
(Multiple Choice)
4.7/5  (35)
(35)
What quantity of NaOH(s) must be added to 1.00 L of 0.200 M HCl to achieve a pH of 12.00? (Assume no volume change.)
(Multiple Choice)
4.7/5  (32)
(32)
Showing 1 - 20 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)