Deck 18: Temperature, Heat, and the First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 18: Temperature, Heat, and the First Law of Thermodynamics
1
If the zeroth law of thermodynamics were not valid, which of the following could not be considered a property of an object?
A) Pressure
B) Center of mass energy
C) Internal energy
D) Momentum
E) Temperature
A) Pressure
B) Center of mass energy
C) Internal energy
D) Momentum
E) Temperature
Temperature
2
There is a temperature at which the reading on the Kelvin scale is numerically:
A) equal to that on the Celsius scale
B) lower than that on the Celsius scale
C) equal to that on the Fahrenheit scale
D) less than zero
E) none of the above
A) equal to that on the Celsius scale
B) lower than that on the Celsius scale
C) equal to that on the Fahrenheit scale
D) less than zero
E) none of the above
equal to that on the Fahrenheit scale
3
A constant-volume gas thermometer is used to measure the temperature of an object. When the thermometer is in contact with water at its triple point (273.16 K) the pressure in the thermometer is 8.500 *104 Pa. When it is in contact with the object the pressure is 9.650 F*104 Pa. The temperature of the object is:
A) 37.0 K
B) 241 K
C) 310 K
D) 314 K
E) 2020 K
A) 37.0 K
B) 241 K
C) 310 K
D) 314 K
E) 2020 K
310 K
4
The international standard thermometer is kept:
A) near Washington, D.C.
B) near Paris, France
C) near the north pole
D) near Rome, Italy
E) nowhere (there is none)
A) near Washington, D.C.
B) near Paris, France
C) near the north pole
D) near Rome, Italy
E) nowhere (there is none)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
Room temperature is about 20 degrees on the:
A) Kelvin scale
B) Celsius scale
C) Fahrenheit scale
D) absolute scale
E) C major scale
A) Kelvin scale
B) Celsius scale
C) Fahrenheit scale
D) absolute scale
E) C major scale

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
Constant-volume gas thermometers using different gases all indicate nearly the same temperature when in contact with the same object if:
A) the volumes are all extremely large
B) the volumes are all the same
C) the pressures are all extremely large
D) the pressures are the same
E) the particle concentrations are all extremely small
A) the volumes are all extremely large
B) the volumes are all the same
C) the pressures are all extremely large
D) the pressures are the same
E) the particle concentrations are all extremely small

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
If two objects are in thermal equilibrium with each other
A) they cannot be moving
B) they cannot be undergoing an elastic collision
C) they cannot have different pressures
D) they cannot be at different temperatures
E) they cannot be falling in the Earth's gravitational field
A) they cannot be moving
B) they cannot be undergoing an elastic collision
C) they cannot have different pressures
D) they cannot be at different temperatures
E) they cannot be falling in the Earth's gravitational field

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following statements is true?
A) temperatures differing by 25 on the Fahrenheit scale must differ by 45 on the Celsius scale
B) 40 K corresponds to -40 C
C) temperatures which differ by 10 on the Celsius scale must differ by 18 on the Fahrenheit scale
D) water at 90 C is warmer than water at 202 F
E) 0 F corresponds to -32 C
A) temperatures differing by 25 on the Fahrenheit scale must differ by 45 on the Celsius scale
B) 40 K corresponds to -40 C
C) temperatures which differ by 10 on the Celsius scale must differ by 18 on the Fahrenheit scale
D) water at 90 C is warmer than water at 202 F
E) 0 F corresponds to -32 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
Suppose object C is in thermal equilibrium with object A and with object B. The zeroth law of thermodynamics states:
A) that C will always be in thermal equilibrium with both A and B
B) that C must transfer energy to both A and B
C) that A is in thermal equilibrium with B
D) that A cannot be in thermal equilibrium with B
E) nothing about the relationship between A and B
A) that C will always be in thermal equilibrium with both A and B
B) that C must transfer energy to both A and B
C) that A is in thermal equilibrium with B
D) that A cannot be in thermal equilibrium with B
E) nothing about the relationship between A and B

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
A thermometer indicates 98.6 C. It may be:
A) outdoors on a cold day
B) in a comfortable room
C) in a cup of hot tea
D) in a normal person's mouth
E) in liquid air
A) outdoors on a cold day
B) in a comfortable room
C) in a cup of hot tea
D) in a normal person's mouth
E) in liquid air

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
The zeroth law of thermodynamics allows us to define
A) work
B) pressure
C) temperature
D) thermal equilibrium
E) internal energy
A) work
B) pressure
C) temperature
D) thermal equilibrium
E) internal energy

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
A balloon is filled with cold air and placed in a warm room. It is NOT in thermal equilibrium with the air of the room until
A) it rises to the ceiling
B) it sinks to the floor
C) it stops expanding
D) it starts to contract
E) none of the above
A) it rises to the ceiling
B) it sinks to the floor
C) it stops expanding
D) it starts to contract
E) none of the above

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
Fahrenheit and Kelvin scales agree numerically at a reading of:
A) -40
B) 0
C) 273
D) 301
E) 574
A) -40
B) 0
C) 273
D) 301
E) 574

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
The air temperature on a summer day might be about:
A) 0 C
B) 10 C
C) 25 C
D) 80 C
E) 125 C
A) 0 C
B) 10 C
C) 25 C
D) 80 C
E) 125 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
When two gases separated by a diathermal wall are in thermal equilibrium with each other:
A) only their pressure must be the same
B) only their volumes must be the same
C) they must have the same number of particles
D) they must have the same pressure and the same volume
E) only their temperatures must be the same
A) only their pressure must be the same
B) only their volumes must be the same
C) they must have the same number of particles
D) they must have the same pressure and the same volume
E) only their temperatures must be the same

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
In constructing a thermometer it is NECESSARY to use a substance that:
A) expands with rising temperature
B) expands linearly with rising temperature
C) will not freeze
D) will not boil
E) undergoes some change when heated or cooled
A) expands with rising temperature
B) expands linearly with rising temperature
C) will not freeze
D) will not boil
E) undergoes some change when heated or cooled

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
When a certain constant volume gas thermometer is in thermal contact with water at its triple point (273.16 K) the pressure is 6.30 * 104 Pa. For this thermometer a kelvin corresponds to a change in pressure of about:
A) 4.34 *102 Pa
B) 2.31 * 102 Pa
C) 1.72 * 103 Pa
D) 2.31 *103 Pa
E) 1.72 * 107 Pa
A) 4.34 *102 Pa
B) 2.31 * 102 Pa
C) 1.72 * 103 Pa
D) 2.31 *103 Pa
E) 1.72 * 107 Pa

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
The "triple point" of a substance is that point for which the temperature and pressure are such that:
A) only solid and liquid are in equilibrium
B) only liquid and vapor are in equilibrium
C) only solid and vapor are in equilibrium
D) solid, liquid and vapor are all in equilibrium
E) the temperature, pressure and density are all numerically equal
A) only solid and liquid are in equilibrium
B) only liquid and vapor are in equilibrium
C) only solid and vapor are in equilibrium
D) solid, liquid and vapor are all in equilibrium
E) the temperature, pressure and density are all numerically equal

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
The diagram shows four thermometers, labeled W, X, Y, and Z. The freezing and boiling points of water are indicated. Rank the thermometers according to the size of a degree on their scales, smallest to largest. 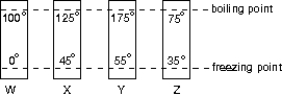
A) W, X, Y, Z
B) Z, Y, X, W
C) Z, Y, W, X
D) Z, X, W, Y
E) W, Y, Z, X

A) W, X, Y, Z
B) Z, Y, X, W
C) Z, Y, W, X
D) Z, X, W, Y
E) W, Y, Z, X

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
A Kelvin thermometer and a Fahrenheit thermometer both give the same reading for a certain sample. The corresponding Celsius temperature is:
A) 574 C
B) 232 C
C) 301 C
D) 614 C
E) 276 C
A) 574 C
B) 232 C
C) 301 C
D) 614 C
E) 276 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
The diagram shows four rectangular plates and their dimensions. All are made of the same material. The temperature now increases. Of these plates: 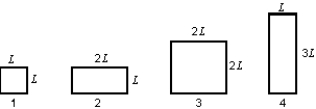
A) the vertical dimension of plate 1 increases the most and the area of plate 1 increases the most
B) the vertical dimension of plate 2 increases the most and the area of plate 4 increases the most
C) the vertical dimension of plate 3 increases the most and the area of plate 1 increases the most
D) the vertical dimension of plate 4 increases the most and the area of plate 3 increases the most
E) the vertical dimension of plate 4 increases the most and the area of plate 4 increases the most

A) the vertical dimension of plate 1 increases the most and the area of plate 1 increases the most
B) the vertical dimension of plate 2 increases the most and the area of plate 4 increases the most
C) the vertical dimension of plate 3 increases the most and the area of plate 1 increases the most
D) the vertical dimension of plate 4 increases the most and the area of plate 3 increases the most
E) the vertical dimension of plate 4 increases the most and the area of plate 4 increases the most

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
A surveyor's 30-m steel tape is correct at 68 F. On a hot day the tape has expanded to 30.01 m. On that day, the tape indicates a distance of 15.52 m between two points. The true distance between these points is:
A) 15.50 m
B) 15.51 m
C) 15.52 m
D) 15.53 m
E) 15.54 m
A) 15.50 m
B) 15.51 m
C) 15.52 m
D) 15.53 m
E) 15.54 m

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
The Stanford linear accelerator contains hundreds of brass disks tightly fitted into a steel tube (see figure). The coefficient of linear expansion of the brass is 2.00 *10-5 per C . The system was assembled by cooling the disks in dry ice (-57 C) to enable them to just slide into the close-fitting tube. If the diameter of a disk is 80.00 mm at 43 C, what is its diameter in the dry ice? 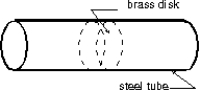
A) 78.40 mm
B) 79.68 mm
C) 80.16 mm
D) 79.84 mm
E) none of these

A) 78.40 mm
B) 79.68 mm
C) 80.16 mm
D) 79.84 mm
E) none of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
The two metallic strips that constitute some thermostats must differ in:
A) length
B) thickness
C) mass
D) rate at which they conduct heat
E) coefficient of linear expansion
A) length
B) thickness
C) mass
D) rate at which they conduct heat
E) coefficient of linear expansion

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
A gram of distilled water at 4 C:
A) will increase slightly in weight when heated to 6 C
B) will decrease slightly in weight when heated to 6 C
C) will increase slightly in volume when heated to 6 C
D) will decrease slightly in volume when heated to 6 C
E) will not change in either volume or weight
A) will increase slightly in weight when heated to 6 C
B) will decrease slightly in weight when heated to 6 C
C) will increase slightly in volume when heated to 6 C
D) will decrease slightly in volume when heated to 6 C
E) will not change in either volume or weight

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
It is more difficult to measure the coefficient of volume expansion of a liquid than that of a solid because:
A) no relation exists between linear and volume expansion coefficients
B) a liquid tends to evaporate
C) a liquid expands too much when heated
D) a liquid expands too little when heated
E) the containing vessel also expands
A) no relation exists between linear and volume expansion coefficients
B) a liquid tends to evaporate
C) a liquid expands too much when heated
D) a liquid expands too little when heated
E) the containing vessel also expands

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
The coefficient of linear expansion of steel is 11 * 10-6 per C . A steel ball has a volume of exactly 100 cm3 at 0 C. When heated to 100 C, its volume becomes:
A) 100.33 cm3
B) 100.0011 cm3
C) 100.0033 cm3
D) 100.000011 cm3
E) none of these
A) 100.33 cm3
B) 100.0011 cm3
C) 100.0033 cm3
D) 100.000011 cm3
E) none of these

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
Heat has the same units as:
A) temperature
B) work
C) energy/time
D) heat capacity
E) energy/volume
A) temperature
B) work
C) energy/time
D) heat capacity
E) energy/volume

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
Heat is:
A) energy transferred by virtue of a temperature difference
B) energy transferred by macroscopic work
C) energy content of an object
D) a temperature difference
E) a property objects have by virtue of their temperatures
A) energy transferred by virtue of a temperature difference
B) energy transferred by macroscopic work
C) energy content of an object
D) a temperature difference
E) a property objects have by virtue of their temperatures

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
The coefficient of linear expansion of iron is 10-5 per C . The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10 C to 60 C?
A) 0.00375 cm3
B) 0.1875 cm3
C) 0.0225 cm3
D) 0.00125 cm3
E) 0.0625 cm3
A) 0.00375 cm3
B) 0.1875 cm3
C) 0.0225 cm3
D) 0.00125 cm3
E) 0.0625 cm3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
When the temperature of a copper penny is increased by 100 C , its diameter increases by 0.17%. The area of one of its faces increases by:
A) 0.17%
B) 0.34%
C) 0.51%
D) 0.13%
E) 0.27%
A) 0.17%
B) 0.34%
C) 0.51%
D) 0.13%
E) 0.27%

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
The mercury column in an ordinary medical thermometer doubles in length when its temperature changes from 95 F to 105 F. Choose the correct statement:
A) the coefficient of volume expansion of mercury is 0.1 per F
B) the coefficient of volume expansion of mercury is 0.3 per F
C) the coefficient of volume expansion of mercury is (0.1/3) per F
D) the vacuum above the column helps to "pull up" the mercury this large amount
E) none of the above is true
A) the coefficient of volume expansion of mercury is 0.1 per F
B) the coefficient of volume expansion of mercury is 0.3 per F
C) the coefficient of volume expansion of mercury is (0.1/3) per F
D) the vacuum above the column helps to "pull up" the mercury this large amount
E) none of the above is true

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
A calorie is about:
A) 0.24 J
B) 8.3 J
C) 250 J
D) 4.2 J
E) 4200 J
A) 0.24 J
B) 8.3 J
C) 250 J
D) 4.2 J
E) 4200 J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
Thin strips of iron and zinc are riveted together to form a bimetallic strip which bends when heated. The iron is on the inside of the bend because:
A) it has a higher coefficient of linear expansion
B) it has a lower coefficient of linear expansion
C) it has a higher specific heat
D) it has a lower specific heat
E) it conducts heat better
A) it has a higher coefficient of linear expansion
B) it has a lower coefficient of linear expansion
C) it has a higher specific heat
D) it has a lower specific heat
E) it conducts heat better

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
The heat capacity of an object is:
A) the amount of heat energy to raise its temperature by 1 C
B) the amount of heat energy to change its state without changing its temperature
C) the amount of heat energy per kilogram to raise its temperature by 1 C
D) the ratio of its specific heat to that of water
E) the change in its temperature caused by adding 1 J of heat
A) the amount of heat energy to raise its temperature by 1 C
B) the amount of heat energy to change its state without changing its temperature
C) the amount of heat energy per kilogram to raise its temperature by 1 C
D) the ratio of its specific heat to that of water
E) the change in its temperature caused by adding 1 J of heat

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Possible units for the coefficient of volume expansion are:
A) mm/C
B) mm3/C
C) (C )3
D) 1/(C )3
E) 1/C
A) mm/C
B) mm3/C
C) (C )3
D) 1/(C )3
E) 1/C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Metal pipes, used to carry water, sometimes burst in the winter because:
A) metal contracts more than water
B) outside of the pipe contracts more than the inside
C) metal becomes brittle when cold
D) ice expands when it melts
E) water expands when it freezes
A) metal contracts more than water
B) outside of the pipe contracts more than the inside
C) metal becomes brittle when cold
D) ice expands when it melts
E) water expands when it freezes

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
The coefficient of expansion of a certain steel is 0.000012 per C . The coefficient of volume expansion, in (C )-1, is:
A) (0.000012)3
B) (4 /3)(0.000012)3
C) 3 * 0.000012
D) 0.000012
E) depends on the shape of the volume to which it will be applied
A) (0.000012)3
B) (4 /3)(0.000012)3
C) 3 * 0.000012
D) 0.000012
E) depends on the shape of the volume to which it will be applied

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
An annular ring of aluminum is cut from an aluminum sheet as shown. When this ring is heated: 
A) the aluminum expands outward and the hole remains the same in size
B) the hole decreases in diameter
C) the area of the hole expands the same percent as any area of the aluminum
D) the area of the hole expands a greater percent than any area of the aluminum
E) linear expansion forces the shape of the hole to be slightly elliptical

A) the aluminum expands outward and the hole remains the same in size
B) the hole decreases in diameter
C) the area of the hole expands the same percent as any area of the aluminum
D) the area of the hole expands a greater percent than any area of the aluminum
E) linear expansion forces the shape of the hole to be slightly elliptical

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
The figure shows a rectangular brass plate at 0 C in which there is cut a rectangular hole of dimensions indicated. If the temperature of the plate is raised to 150 C: 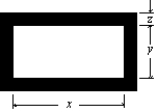
A) x will increase and y will decrease
B) both x and y will decrease
C) x will decrease and y will increase
D) both x and y will increase
E) the changes in x and y depend on the dimension z

A) x will increase and y will decrease
B) both x and y will decrease
C) x will decrease and y will increase
D) both x and y will increase
E) the changes in x and y depend on the dimension z

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
For constant volume processes the heat capacity of gas A is greater than the heat capacity of gas B. We conclude that when they both absorb the same energy as heat at constant volume:
A) the temperature of A increases more than the temperature of B
B) the temperature of B increases more than the temperature of A
C) the internal energy of A increases more than the internal energy of B
D) the internal energy of B increases more than the internal energy of A
E) A does more positive work than B
A) the temperature of A increases more than the temperature of B
B) the temperature of B increases more than the temperature of A
C) the internal energy of A increases more than the internal energy of B
D) the internal energy of B increases more than the internal energy of A
E) A does more positive work than B

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
The specific heat of a substance is:
A) the amount of heat energy to change the state of one gram of the substance
B) the amount of heat energy per unit mass emitted by oxidizing the substance
C) the amount of heat energy per unit mass to raise the substance from its freezing to its boiling point
D) the amount of heat energy per unit mass to raise the temperature of the substance by 1 C
E) the temperature of the object divided by its mass
A) the amount of heat energy to change the state of one gram of the substance
B) the amount of heat energy per unit mass emitted by oxidizing the substance
C) the amount of heat energy per unit mass to raise the substance from its freezing to its boiling point
D) the amount of heat energy per unit mass to raise the temperature of the substance by 1 C
E) the temperature of the object divided by its mass

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
Ten grams of ice at -20 C is to be changed to steam at 130 C. The specific heat of both ice and steam is 0.5 cal/g . C. The heat of fusion is 80 cal/g and the heat of vaporization is 540 cal/g. The entire process requires:
A) 750 cal
B) 1250 cal
C) 6950 cal
D) 7450 cal
E) 7700 cal
A) 750 cal
B) 1250 cal
C) 6950 cal
D) 7450 cal
E) 7700 cal

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
Solid A, with mass M, is at its melting point TA. It is placed in thermal contact with solid B, with heat capacity CB and initially at temperature TB (TB > TA). The combination is thermally isolated. A has latent heat of fusion L and when it has melted has heat capacity CA. If A completely melts the final temperature of both A and B is:
A) (CATA + CBTB - ML)/(CA + CB)
B) (CATA - CBTB + ML)/(CA + CB)
C) (CATA + CBTB + ML)/(CA - CB)
D) (CATA + CBTB + ML)/(CA)
A) (CATA + CBTB - ML)/(CA + CB)
B) (CATA - CBTB + ML)/(CA + CB)
C) (CATA + CBTB + ML)/(CA - CB)
D) (CATA + CBTB + ML)/(CA)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
Steam at 1 atm and 100 C enters a radiator and leaves as water (at 1 atm and 80 C). Take the heat of vaporization to be 540 cal/g. Of the total energy given off as heat, what percent arises from the cooling of the water?
A) 100
B) 54
C) 26
D) 14
E) 3.6
A) 100
B) 54
C) 26
D) 14
E) 3.6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
The specific heat of lead is 0.030 cal/g . C. 300 g of lead shot at 100 C is mixed with 100 g of water at 70 C in an insulated container. The final temperature of the mixture is:
A) 100 C
B) 85.5 C
C) 79.5 C
D) 74.5 C
E) 72.5 C
A) 100 C
B) 85.5 C
C) 79.5 C
D) 74.5 C
E) 72.5 C

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
During the time that latent heat is involved in a change of state:
A) the temperature does not change
B) the substance always expands
C) a chemical reaction takes place
D) molecular activity remains constant
E) kinetic energy changes into potential energy
A) the temperature does not change
B) the substance always expands
C) a chemical reaction takes place
D) molecular activity remains constant
E) kinetic energy changes into potential energy

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
The heat capacity at constant volume and the heat capacity at constant pressure have different values because:
A) heat increases the internal energy at constant volume but not at constant pressure
B) heat increases the internal energy at constant pressure but not at constant volume
C) the system does work at constant volume but not at constant pressure
D) the system does work at constant pressure but not at constant volume
E) the system does more work at constant volume than at constant pressure
A) heat increases the internal energy at constant volume but not at constant pressure
B) heat increases the internal energy at constant pressure but not at constant volume
C) the system does work at constant volume but not at constant pressure
D) the system does work at constant pressure but not at constant volume
E) the system does more work at constant volume than at constant pressure

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
How many calories are required to change one gram of 0 C ice to 100 C steam? The latent heat of fusion is 80 cal/g and the latent heat of vaporization is 540 cal/g. The specific heat of water is 1.00 cal/g . K.
A) 100
B) 540
C) 620
D) 720
E) 900
A) 100
B) 540
C) 620
D) 720
E) 900

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
Two different samples have the same mass and temperature. Equal quantities of energy are absorbed as heat by each. Their final temperatures may be different because the samples have different:
A) thermal conductivities
B) coefficients of expansion
C) densities
D) volumes
E) heat capacities
A) thermal conductivities
B) coefficients of expansion
C) densities
D) volumes
E) heat capacities

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Take the mechanical equivalent of heat as 4 J/cal. A 10-gram bullet moving at 2000 m/s plunges into 1 kg of paraffin wax (specific heat 0.7 cal/g. C). The wax was initially at 20 C. Assuming that all the bullet's energy heats the wax, its final temperature ( C) is:
A) 20.14
B) 23.5
C) 20.006
D) 27.1
E) 30.23
A) 20.14
B) 23.5
C) 20.006
D) 27.1
E) 30.23

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
A heat of transformation of a substance is:
A) the energy absorbed as heat during a phase transformation
B) the energy per unit mass absorbed as heat during a phase transformation
C) the same as the heat capacity
D) the same as the specific heat
E) the same as the molar specific heat
A) the energy absorbed as heat during a phase transformation
B) the energy per unit mass absorbed as heat during a phase transformation
C) the same as the heat capacity
D) the same as the specific heat
E) the same as the molar specific heat

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
Object A, with heat capacity CA and initially at temperature TA, is placed in thermal contact with object B, with heat capacity CB and initially at temperature TB. The combination is thermally isolated. If the heat capacities are independent of the temperature and no phase changes occur, the final temperature of both objects is:
A) (CATA - CBTB)/(CA + CB)
B) (CATA + CBTB)/(CA + CB)
C) (CATA - CBTB)/(CA - CB)
D) (CA - CB) TA - TB
E) (CA + CB) TA - TB
A) (CATA - CBTB)/(CA + CB)
B) (CATA + CBTB)/(CA + CB)
C) (CATA - CBTB)/(CA - CB)
D) (CA - CB) TA - TB
E) (CA + CB) TA - TB

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
The same energy Q enters five different substances as heat.
A) The temperature of 3 g of substance A by 10 K
B) The temperature of 4 g of substance B by 4 K
C) The temperature of 6 g of substance C by 15 K
D) The temperature of 8 g of substance D by 5 K
E) The temperature of 10 g of substance E by 10 K Which of these has the greatest specific heat?
A) The temperature of 3 g of substance A by 10 K
B) The temperature of 4 g of substance B by 4 K
C) The temperature of 6 g of substance C by 15 K
D) The temperature of 8 g of substance D by 5 K
E) The temperature of 10 g of substance E by 10 K Which of these has the greatest specific heat?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
The energy given off by 300 grams of an alloy as it cools through 50 C raises the temperature of 300 grams of water from 30 C to 40 C. The specific heat of the alloy (in cal/g C˚) is:
A) 0.015
B) 0.10
C) 0.15
D) 0.20
E) 0.50
A) 0.015
B) 0.10
C) 0.15
D) 0.20
E) 0.50

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
An insulated container, filled with water, contains a thermometer and a paddle wheel. The paddle wheel can be rotated by an external source. This apparatus can be used to determine:
A) specific heat of water
B) relation between kinetic energy and absolute temperature
C) thermal conductivity of water
D) efficiency of changing work into heat
E) mechanical equivalent of heat
A) specific heat of water
B) relation between kinetic energy and absolute temperature
C) thermal conductivity of water
D) efficiency of changing work into heat
E) mechanical equivalent of heat

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
The heat of fusion of water is 79.5 cal/g. This means 80 cal of energy are required to:
A) raise the temperature of 1 kg of water by 1 K
B) turn 1 kg of water to steam
C) raise the temperature of 1 kg of ice by 1 K
D) melt 1 kg of ice
E) increase the internal energy of 1 kg of water by 1 kJ
A) raise the temperature of 1 kg of water by 1 K
B) turn 1 kg of water to steam
C) raise the temperature of 1 kg of ice by 1 K
D) melt 1 kg of ice
E) increase the internal energy of 1 kg of water by 1 kJ

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
The heat capacity of object B is twice that of object A. Initially A is at 300 K and B is at 450 K. They are placed in thermal contact and the combination is isolated. The final temperature of both objects is:
A) 200 K
B) 300 K
C) 400 K
D) 450 K
E) 600 K
A) 200 K
B) 300 K
C) 400 K
D) 450 K
E) 600 K

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
The formation of ice from water is accompanied by:
A) absorption of energy as heat
B) temperature increase
C) decrease in volume
D) an evolution of heat
E) temperature decrease
A) absorption of energy as heat
B) temperature increase
C) decrease in volume
D) an evolution of heat
E) temperature decrease

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
A cube of aluminum has an edge length of 20 cm. Aluminum has a density 2.7 times that of water (1 g/cm3) and a specific heat 0.217 times that of water (1 cal/g.C˚). When the internal energy of the cube increases by 47000 cal its temperature increases by:
A) 5 C˚
B) 10 C˚
C) 20 C˚
D) 100 C˚
E) 200 C˚
A) 5 C˚
B) 10 C˚
C) 20 C˚
D) 100 C˚
E) 200 C˚

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
In an adiabatic process:
A) the energy absorbed as heat equals the work done by the system on its environment
B) the energy absorbed as heat equals the work done by the environment on the system
C) the energy absorbed as heat equals the change in internal energy
D) the work done by the environment on the system equals the change in internal energy
E) the work done by the system on its environment equals to the change in internal energy
A) the energy absorbed as heat equals the work done by the system on its environment
B) the energy absorbed as heat equals the work done by the environment on the system
C) the energy absorbed as heat equals the change in internal energy
D) the work done by the environment on the system equals the change in internal energy
E) the work done by the system on its environment equals to the change in internal energy

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
A metal sample of mass M requires a power input P to just remain molten. When the heater is turned off, the metal solidifies in a time T. The specific latent heat of fusion of this metal is:
A) P/MT
B) T/PM
C) PM/T
D) PMT
E) PT/M
A) P/MT
B) T/PM
C) PM/T
D) PMT
E) PT/M

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
Of the following which might NOT vanish over one cycle of a cyclic process?
A) the change in the internal energy of the substance
B) the change in pressure of the substance
C) the work done by the substance
D) the change in the volume of the substance
E) the change in the temperature of the substance
A) the change in the internal energy of the substance
B) the change in pressure of the substance
C) the work done by the substance
D) the change in the volume of the substance
E) the change in the temperature of the substance

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
The rate of heat flow by condition through a slab does NOT depend upon the:
A) temperature difference between opposite faces of the slab
B) thermal conductivity of the slab
C) slab thickness
D) cross-sectional area of the slab
E) specific heat of the slab
A) temperature difference between opposite faces of the slab
B) thermal conductivity of the slab
C) slab thickness
D) cross-sectional area of the slab
E) specific heat of the slab

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
Pressure vs. volume graphs for a certain gas undergoing five different cyclic processes are shown below. During which cycle does the gas do the greatest positive work? 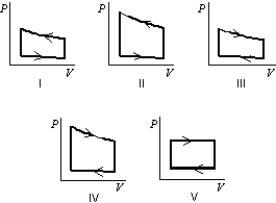
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
A system undergoes an adiabatic process in which its internal energy increases by 20 J. Which of the following statements is true?
A) 20 J of work was done on the system
B) 20 J of work was done by the system
C) the system received 20 J of energy as heat
D) the system lost 20 J of energy as heat
E) none of the above are true
A) 20 J of work was done on the system
B) 20 J of work was done by the system
C) the system received 20 J of energy as heat
D) the system lost 20 J of energy as heat
E) none of the above are true

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Fifty grams of ice at 0 C is placed in a thermos bottle containing one hundred grams of water at 6 C. How many grams of ice will melt? The heat of fusion of water is 333 kJ/kg and the specific heat is 4190 J/kg .K.
A) 7.5
B) 2.0
C) 8.3
D) 17
E) 50
A) 7.5
B) 2.0
C) 8.3
D) 17
E) 50

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
To help keep buildings cool in the summer, dark colored window shades have been replaced by light colored shades. This is because light colored shades:
A) are more pleasing to the eye
B) absorb more sunlight
C) reflect more sunlight
D) transmit more sunlight
E) have a lower thermal conductivity
A) are more pleasing to the eye
B) absorb more sunlight
C) reflect more sunlight
D) transmit more sunlight
E) have a lower thermal conductivity

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
The rate of heat flow through a slab is Pcond. If the slab thickness is doubled, its cross-sectional area is halved, and the temperature difference across it is doubled, then the rate of heat flow becomes:
A) 2Pcond
B) Pcond/2
C) Pcond
D) Pcond/8
E) 8Pcond
A) 2Pcond
B) Pcond/2
C) Pcond
D) Pcond/8
E) 8Pcond

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
Inside a room at a uniform comfortable temperature, metallic objects generally feel cooler to the touch than wooden objects do. This is because:
A) a given mass of wood contains more heat than the same mass of metal
B) metal conducts heat better than wood
C) heat tends to flow from metal to wood
D) the equilibrium temperature of metal in the room is lower than that of wood
E) the human body, being organic, resembles wood more closely than it resembles metal
A) a given mass of wood contains more heat than the same mass of metal
B) metal conducts heat better than wood
C) heat tends to flow from metal to wood
D) the equilibrium temperature of metal in the room is lower than that of wood
E) the human body, being organic, resembles wood more closely than it resembles metal

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
In a certain process a gas ends in its original thermodynamic state. Of the following, which is possible as the net result of the process?
A) It is adiabatic and the gas does 50 J of work
B) The gas does no work but absorbs 50 J of energy as heat
C) The gas does no work but rejects 50 J of energy as heat
D) The gas rejects 50 J of heat and does 50 J of work
E) The gas absorbs 50 J of energy as heat and does 50 J of work
A) It is adiabatic and the gas does 50 J of work
B) The gas does no work but absorbs 50 J of energy as heat
C) The gas does no work but rejects 50 J of energy as heat
D) The gas rejects 50 J of heat and does 50 J of work
E) The gas absorbs 50 J of energy as heat and does 50 J of work

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements pertaining to a vacuum flask (thermos) is NOT correct?
A) Silvering reduces radiation loss
B) Vacuum reduces conduction loss
C) Vacuum reduces convection loss
D) Vacuum reduces radiation loss
E) Glass walls reduce conduction loss
A) Silvering reduces radiation loss
B) Vacuum reduces conduction loss
C) Vacuum reduces convection loss
D) Vacuum reduces radiation loss
E) Glass walls reduce conduction loss

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
The units of thermal conductivity might be:
A) cal . cm/(s . C )
B) cal/(cm. s .C )
C) cal. s/(cm.C )
D) cm . s . C /cal
E) C /(cal . cm .s)
A) cal . cm/(s . C )
B) cal/(cm. s .C )
C) cal. s/(cm.C )
D) cm . s . C /cal
E) C /(cal . cm .s)

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
The diagram shows four slabs of different materials with equal thickness, placed side by side. Heat flows from left to right and the steady-state temperatures of the interfaces are given. Rank the materials according to their thermal conductivities, smallest to largest. 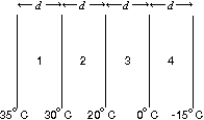
A) 1, 2, 3, 4
B) 2, 1, 3, 4
C) 3, 4, 1, 2
D) 3, 4, 2, 1
E) 4, 3, 2, 1

A) 1, 2, 3, 4
B) 2, 1, 3, 4
C) 3, 4, 1, 2
D) 3, 4, 2, 1
E) 4, 3, 2, 1

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
An iron stove, used for heating a room by radiation, is more efficient if:
A) its inner surface is highly polished
B) its inner surface is covered with aluminum paint
C) its outer surface is covered with aluminum paint
D) its outer surface is rough and black
E) its outer surface is highly polished
A) its inner surface is highly polished
B) its inner surface is covered with aluminum paint
C) its outer surface is covered with aluminum paint
D) its outer surface is rough and black
E) its outer surface is highly polished

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
Of the following which might NOT vanish over one cycle of a cyclic process?
A) at wor done by the substance minus the energy absorbed by the substance as heat
B) the change in the pressure of the substance
C) the energy absorbed by the substance as heat
D) the change in the volume of the substance
E) the change in the temperature of the substance
A) at wor done by the substance minus the energy absorbed by the substance as heat
B) the change in the pressure of the substance
C) the energy absorbed by the substance as heat
D) the change in the volume of the substance
E) the change in the temperature of the substance

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
A slab of material has area A, thickness L, and thermal conductivity k. One of its surfaces (P) is maintained at temperature T1 and the other surface (Q) is maintained at a lower temperature T2. The rate of heat flow from P to Q is:
A) kA(T1 - T2)/L2
B) kL(T1 - T2)/A
C) kA(T1 - T2)/L
D) k(T1 - T2)/(LA)
E) LA(T1 - T2)/k
A) kA(T1 - T2)/L2
B) kL(T1 - T2)/A
C) kA(T1 - T2)/L
D) k(T1 - T2)/(LA)
E) LA(T1 - T2)/k

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20 C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg .K.How many joules of energy per minute does this humidifier require?
A) 4800
B) 18,600
C) 16,200
D) 24,600
E) 2400
A) 4800
B) 18,600
C) 16,200
D) 24,600
E) 2400

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
During an adiabatic process an object does 100 J of work and its temperature decreases by 5 K. During another process it does 25 J of work and its temperature decreases by 5 K. Its heat capacity for the second process is:
A) 20 J/K
B) 24 J/JK
C) 5 J/K
D) 15 J/K
E) 100 K/J
A) 20 J/K
B) 24 J/JK
C) 5 J/K
D) 15 J/K
E) 100 K/J

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
According to the first law of thermodynamics, applied to a gas, the increase in the internal energy during any process:
A) equals the heat input minus the work done on the gas
B) equals the heat input plus the work done on the gas
C) equals the work done on the gas minus the heat input
D) is independent of the heat input
E) is independent of the work done on the gas
A) equals the heat input minus the work done on the gas
B) equals the heat input plus the work done on the gas
C) equals the work done on the gas minus the heat input
D) is independent of the heat input
E) is independent of the work done on the gas

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


