Exam 18: Temperature, Heat, and the First Law of Thermodynamics
Exam 1: Measurement31 Questions
Exam 2: Motion Along a Straight Line79 Questions
Exam 3: Vector39 Questions
Exam 4: Motion in Two and Three Dimensions47 Questions
Exam 5: Force and Motion I68 Questions
Exam 6: Force and Motion II71 Questions
Exam 7: Kinetic Energy and Work67 Questions
Exam 8: Potential Energy and Conservation of Energy61 Questions
Exam 9: Center of Mass and Linear Momentum81 Questions
Exam 10: Rotation82 Questions
Exam 11: Rolling, Torque, and Angular Momentum54 Questions
Exam 12: Equilibrium and Elasticity53 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids85 Questions
Exam 15: Oscillations62 Questions
Exam 16: Waves I71 Questions
Exam 17: Waves II61 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics82 Questions
Exam 19: The Kinetic Theory of Gases95 Questions
Exam 20: Entropy and the Second Law of Thermodynamics56 Questions
Exam 21: Electric Charge45 Questions
Exam 22: Electric Fields49 Questions
Exam 23: Gauss Law34 Questions
Exam 24: Electric Potential44 Questions
Exam 25: Capacitance55 Questions
Exam 26: Current and Resistance49 Questions
Exam 27: Circuits70 Questions
Exam 28: Magnetic Fields48 Questions
Exam 29: Magnetic Fields Due to Currents47 Questions
Exam 30: Induction and Inductance85 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current84 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves79 Questions
Exam 34: Images72 Questions
Exam 35: Interference40 Questions
Exam 36: Diffraction74 Questions
Exam 37: Relativity65 Questions
Exam 38: Photons and Matter Waves53 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms76 Questions
Exam 41: Conduction of Electricity in Solids48 Questions
Exam 42: Nuclear Physics67 Questions
Exam 43: Energy From the Nucleus44 Questions
Exam 44: Quarks, Leptons, and the Big Bang52 Questions
Select questions type
The diagram shows four thermometers, labeled W, X, Y, and Z. The freezing and boiling points of water are indicated. Rank the thermometers according to the size of a degree on their scales, smallest to largest. 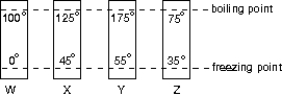
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
D
The specific heat of lead is 0.030 cal/g . C. 300 g of lead shot at 100 C is mixed with 100 g of water at 70 C in an insulated container. The final temperature of the mixture is:
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
E
The coefficient of linear expansion of steel is 11 * 10-6 per C . A steel ball has a volume of exactly 100 cm3 at 0 C. When heated to 100 C, its volume becomes:
(Multiple Choice)
4.8/5  (35)
(35)
Thin strips of iron and zinc are riveted together to form a bimetallic strip which bends when heated. The iron is on the inside of the bend because:
(Multiple Choice)
4.8/5  (29)
(29)
A balloon is filled with cold air and placed in a warm room. It is NOT in thermal equilibrium with the air of the room until
(Multiple Choice)
4.9/5  (45)
(45)
Object A, with heat capacity CA and initially at temperature TA, is placed in thermal contact with object B, with heat capacity CB and initially at temperature TB. The combination is thermally isolated. If the heat capacities are independent of the temperature and no phase changes occur, the final temperature of both objects is:
(Multiple Choice)
4.9/5  (37)
(37)
The heat capacity of object B is twice that of object A. Initially A is at 300 K and B is at 450 K. They are placed in thermal contact and the combination is isolated. The final temperature of both objects is:
(Multiple Choice)
4.8/5  (44)
(44)
A metal sample of mass M requires a power input P to just remain molten. When the heater is turned off, the metal solidifies in a time T. The specific latent heat of fusion of this metal is:
(Multiple Choice)
4.9/5  (36)
(36)
When two gases separated by a diathermal wall are in thermal equilibrium with each other:
(Multiple Choice)
4.7/5  (35)
(35)
Of the following which might NOT vanish over one cycle of a cyclic process?
(Multiple Choice)
4.7/5  (29)
(29)
The diagram shows four rectangular plates and their dimensions. All are made of the same material. The temperature now increases. Of these plates: 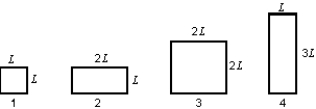
(Multiple Choice)
4.9/5  (31)
(31)
Fifty grams of ice at 0 C is placed in a thermos bottle containing one hundred grams of water at 6 C. How many grams of ice will melt? The heat of fusion of water is 333 kJ/kg and the specific heat is 4190 J/kg .K.
(Multiple Choice)
4.8/5  (38)
(38)
The diagram shows four slabs of different materials with equal thickness, placed side by side. Heat flows from left to right and the steady-state temperatures of the interfaces are given. Rank the materials according to their thermal conductivities, smallest to largest. 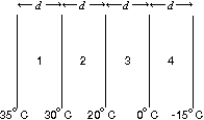
(Multiple Choice)
4.9/5  (41)
(41)
In a certain process a gas ends in its original thermodynamic state. Of the following, which is possible as the net result of the process?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 1 - 20 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)