Deck 19: Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 19: Amines
1
Provide the structure of N-ethyl-1-butanamine.
CH3CH2CH2CH2NHCH2CH3
2
Name the compound shown below. 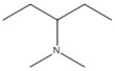

N,N-dimethyl-3-pentanamine or N,N-dimethylpentan-3-amine
3
Provide the name of the compound shown below. 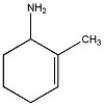

6-amino-1-methylcyclohexene
4
Provide the name of (CH3CH2)4N+ Cl-.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
Provide the systematic name for the compound below. 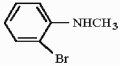


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
Name the compound shown below. 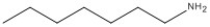


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the IUPAC name for the compound shown below. 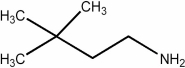


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
Provide the structure of 2,4-dimethylpyridine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
Draw the structure of piperidine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
Draw the structure of 3-pentanamine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
The structure of morphine is shown below. The amine in morphine is classified as ________. 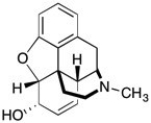
A) 1°
B) 2°
C) 3°
D) aryl

A) 1°
B) 2°
C) 3°
D) aryl

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
Draw the structure of N-propyl-4-methyl-3-octanamine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Provide the systematic name for the compound below. 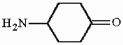


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the correct systematic name for the following compound. 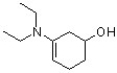
A) N,N-diethyl-5-hydroxycyclohex-1-enamine
B) N,N-diethyl-4-hydroxycyclohex-1-en-2-amine
C) 3-(diethylamino)cyclohex-3-enol
D) 2-(diethylamino)cyclohex-1-en-4-ol

A) N,N-diethyl-5-hydroxycyclohex-1-enamine
B) N,N-diethyl-4-hydroxycyclohex-1-en-2-amine
C) 3-(diethylamino)cyclohex-3-enol
D) 2-(diethylamino)cyclohex-1-en-4-ol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
Provide the name for the compound below. 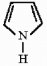


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
Draw the structure of N-ethyl-N-methyl-1-hexanamine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
Provide the systematic name for the compound below.
(CH3)4N+Cl-
(CH3)4N+Cl-

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
sec-Butylamine is the common name of what compound?
A) 1-butanamine
B) 2-butanamine
C) N-methyl-1-propanamine
D) N-methyl-2-propanamine
E) N-ethylethanamine
A) 1-butanamine
B) 2-butanamine
C) N-methyl-1-propanamine
D) N-methyl-2-propanamine
E) N-ethylethanamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
Provide the IUPAC name of (CH3)3CCH2CH2NHCH2CH3.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Draw the structure of 2,3-dimethyl-2-butanamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
Rank the following compounds in order of increasing boiling point (lowest to highest): triethylamine, cyclohexylamine, di-n-propylamine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds contains a tertiary amine?
1) 2-methyl-2-propanamine
2) N-ethylpyrrolidine
3) N-methylcyclohexylamine
4) 2-(dimethylamino)butanal
1) 2-methyl-2-propanamine
2) N-ethylpyrrolidine
3) N-methylcyclohexylamine
4) 2-(dimethylamino)butanal

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a secondary amine?
A) cyclohexylamine
B) 3-pentanamine
C) methylamine
D) N,N-dimethylaniline
E) N-ethyl-1-propanamine
A) cyclohexylamine
B) 3-pentanamine
C) methylamine
D) N,N-dimethylaniline
E) N-ethyl-1-propanamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a tertiary amine?
A) cyclohexylamine
B) 3-pentanamine
C) methylamine
D) N,N-dimethylaniline
E) N-ethyl-1-propanamine
A) cyclohexylamine
B) 3-pentanamine
C) methylamine
D) N,N-dimethylaniline
E) N-ethyl-1-propanamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds is a 2° amine?
A) cyclohexylamine
B) t-butylamine
C) N-ethylaniline
D) N,N-diethylaniline
E) N-butylpyridinium bromide
A) cyclohexylamine
B) t-butylamine
C) N-ethylaniline
D) N,N-diethylaniline
E) N-butylpyridinium bromide

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the correct name for the following structure. 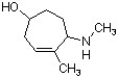
A) 5-methyl-4-(methylamino)cyclohept-5-enol
B) 5-hydroxy-2,N-dimethylcyclohept-2-enamine
C) 4-hydroxy-7,N-dimethylcyclohept-6-enamine
D) 4-methyl-5-(methylamino)cyclohept-3-enol

A) 5-methyl-4-(methylamino)cyclohept-5-enol
B) 5-hydroxy-2,N-dimethylcyclohept-2-enamine
C) 4-hydroxy-7,N-dimethylcyclohept-6-enamine
D) 4-methyl-5-(methylamino)cyclohept-3-enol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
What common names for 1,4-butanediamine and 1,5-pentanediamine are derived from their characteristic pungent odors?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
Which has the higher boiling point, butan-1-ol or 1-butanamine? Explain briefly.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
Provide the correct IUPAC name for the following amine. 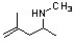
A) 4-(methylamino)-2-methyl-1-pentene
B) 2,N-dimethyl-1-penten-4-amine
C) 4,N-dimethyl-4-penten-2-amine
D) 1,3,N-trimethyl-3-buten-1-amine

A) 4-(methylamino)-2-methyl-1-pentene
B) 2,N-dimethyl-1-penten-4-amine
C) 4,N-dimethyl-4-penten-2-amine
D) 1,3,N-trimethyl-3-buten-1-amine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
Arrange the following compounds in order of increasing boiling point:
ethyl methyl ether, 1-propanol, N-methylethanamine, and 1-propanamine.
ethyl methyl ether, 1-propanol, N-methylethanamine, and 1-propanamine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
Why can 1,2,2-trimethylaziridine be resolved into enantiomers while 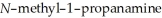
cannot?
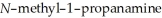
cannot?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
Arrange the following in order of increasing basicity:
aniline, p-nitroaniline, p-toluidine, and p-methoxyaniline.
aniline, p-nitroaniline, p-toluidine, and p-methoxyaniline.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
Circle all of the following structures that can be resolved into pure enantiomers. 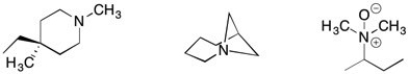


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following amines can be resolved into enantiomers?
A) trimethylamine
B) 3-pentanamine
C) 2-pentanamine
D) dimethylammonium chloride
E) 4-(dimethylamino) pyridine
A) trimethylamine
B) 3-pentanamine
C) 2-pentanamine
D) dimethylammonium chloride
E) 4-(dimethylamino) pyridine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
Provide the product of the reaction shown below. 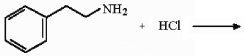
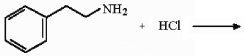

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
Arrange the following compounds in order of increasing boiling point:
CH3NHCH2CH3, CH3OCH2CH3, (CH3)3N, and CH3CH2CH2OH.
CH3NHCH2CH3, CH3OCH2CH3, (CH3)3N, and CH3CH2CH2OH.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
Name the compound shown below. 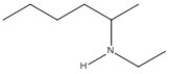


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following amines is most soluble in water?
A) pyrrolidine
B) PhNH2
C) (CH3)3N
D) (CH3CH2CH2)2NH
E) ethylamine
A) pyrrolidine
B) PhNH2
C) (CH3)3N
D) (CH3CH2CH2)2NH
E) ethylamine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
The nitrogen atom of trimethylamine is ________ hybridized which is reflected in the CNC bond angle of ________.
A) sp; 180°
B) sp2; 120°
C) sp2; 108°
D) sp3; 120°
E) sp3; 108°
A) sp; 180°
B) sp2; 120°
C) sp2; 108°
D) sp3; 120°
E) sp3; 108°

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
The nitrogen's lone pair in pyrrolidine is best described as occupying what type of orbital?
A) s
B) sp3
C) sp2
D) sp
E) p
A) s
B) sp3
C) sp2
D) sp
E) p

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Physostigmine is used in the treatment of glaucoma. Within this structure, the atom indicated by ________ is most basic, while atom ________ is least basic. 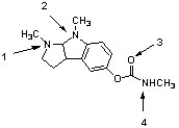
A) 1 (most basic), 4 (least basic)
B) 1 (most basic), 3 (least basic)
C) 2 (most basic), 4 (least basic)
D) 2 (most basic), 3 (least basic)
E) None of the above

A) 1 (most basic), 4 (least basic)
B) 1 (most basic), 3 (least basic)
C) 2 (most basic), 4 (least basic)
D) 2 (most basic), 3 (least basic)
E) None of the above

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
When aniline is treated with molecular bromine in the presence of a mild base (NaHCO3), polyhalogenation occurs. However, when aniline is treated with molecular bromine in the presence of a strong Lewis Acid like FeBr3, no reaction takes place. Briefly explain this difference in reactivity.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Circle the stronger base in the pair below, and briefly explain your choice. 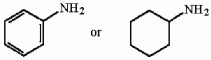


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
How might one distinguish a sample of n-butylamine from a sample of diethylamine using IR spectroscopy?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
When pentanal reacts with ethylamine under conditions of acid catalysis, the major organic product is ________.
A) a ketone
B) a nitrile
C) an imine
D) an oxime
E) a hydrazone
A) a ketone
B) a nitrile
C) an imine
D) an oxime
E) a hydrazone

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Circle the stronger base in the pair below, and briefly explain your choice. 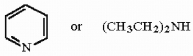
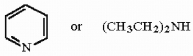

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
How might one distinguish a sample of trimethylamine from a sample of 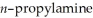
using IR spectroscopy?
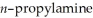
using IR spectroscopy?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
In the mass spectrum of compounds with one nitrogen ________.
A) most fragments are odd and the molecular ion is even
B) most fragments are odd and the molecular ion is odd
C) most fragments are even and the molecular ion is even
D) most fragments are even and the molecular ion is odd
A) most fragments are odd and the molecular ion is even
B) most fragments are odd and the molecular ion is odd
C) most fragments are even and the molecular ion is even
D) most fragments are even and the molecular ion is odd

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following amines is most basic?
A) aniline
B) N-ethylaniline
C) N,N-diethylaniline
D) piperidine
E) pyrrole
A) aniline
B) N-ethylaniline
C) N,N-diethylaniline
D) piperidine
E) pyrrole

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
When CH3CH2CHO reacts with PhNHNH2 under conditions of acid catalysis, the major organic product is ________.
A) a ketone
B) a nitrile
C) an imine
D) an oxime
E) a hydrazone
A) a ketone
B) a nitrile
C) an imine
D) an oxime
E) a hydrazone

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
A three-carbon, nitrogen-containing compound exhibits 3 13C NMR peaks (d 11.2, 27.3, and 44.9). Which of the following compounds best matches this spectral data?
A) H2NCH2CH2CH2OH
B) (CH3)2CHNH2
C) CH3NHCH2CH3
D) CH3CH2CH2NH2
E) CH3CH2C≡N
A) H2NCH2CH2CH2OH
B) (CH3)2CHNH2
C) CH3NHCH2CH3
D) CH3CH2CH2NH2
E) CH3CH2C≡N

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Predict the product of the reaction shown below and add arrows to show the mechanism. 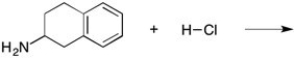


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
In 1H NMR protons on the α-carbon of amines typically appear in the range of ________.
A) 1.0 and 2.0 ppm
B) 2.0 and 3.0 ppm
C) 3.0 and 4.0 ppm
D) 6.0 and 7.0 ppm
E) 9.0 and 10.0 ppm
A) 1.0 and 2.0 ppm
B) 2.0 and 3.0 ppm
C) 3.0 and 4.0 ppm
D) 6.0 and 7.0 ppm
E) 9.0 and 10.0 ppm

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following amines is the strongest base?
A) cyclohexylamine
B) pyrrole
C) p-iodoaniline
D) piperidine
E) imidazole
A) cyclohexylamine
B) pyrrole
C) p-iodoaniline
D) piperidine
E) imidazole

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
How might the presence of an N-H functionally be indicated using NMR spectroscopy?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
Since alkyl groups are electron donors, one might conclude that tertiary amines are much more basic than primary amines. Actually, primary and tertiary amines show similar ranges of basicity. Offer an explanation.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
The α-carbon atom bonded to the nitrogen of an alkylamine usually appears in what chemical shift (δ) range?
A) 5-20
B) 30-50
C) 80-100
D) 120-150
E) 180-220
A) 5-20
B) 30-50
C) 80-100
D) 120-150
E) 180-220

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
Circle the stronger base in the pair below, and briefly explain your choice. 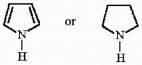
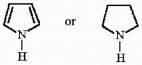

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
A sample of cyclohexylamine is contaminated with considerable hydrocarbon impurity. How might the sample be purified using a technique based on solubility differences?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
The following structure has been used in monitoring the development of amyloid plaques in Alzheimer's patients (J. Med. Chem. 2011, 949). Which sequence correctly ranks the following nitrogens in order of increasing pKb value? 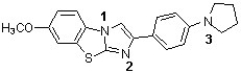
A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

A) 2 < 3 < 1
B) 3 < 2 < 1
C) 2 < 1 < 3
D) 1 < 3 < 2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
Pyridine typically undergoes electrophilic aromatic substitution ________ rapidly than benzene, and its preferred site of substitution is the ________ - position.
A) more; 2
B) more; 3
C) more; 4
D) less; 2
E) less; 3
A) more; 2
B) more; 3
C) more; 4
D) less; 2
E) less; 3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
Complete the following reaction by filling in the necessary reagents. 


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the major organic product of the following reaction. 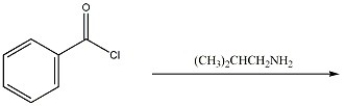


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the major organic compound in the following reaction. 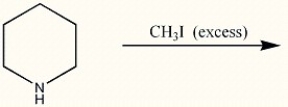
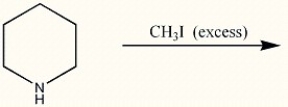

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
When pyridine undergoes electrophilic aromatic substitution, at which position does substitution occur?
A) 2-position
B) 3-position
C) 4-position
D) At all positions equally
A) 2-position
B) 3-position
C) 4-position
D) At all positions equally

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
What compound results from the reaction below? 
A) imine
B) amide
C) primary amine
D) quaternary ammonium salt
E) nitrous acid

A) imine
B) amide
C) primary amine
D) quaternary ammonium salt
E) nitrous acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the structure of the major organic product in the reaction below. 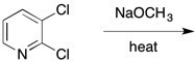
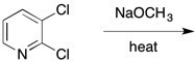

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
Why are strongly acidic reagents inappropriate when attempting an electrophilic aromatic substitution with aniline?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the structure of the major organic product in the reaction below. 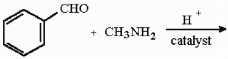
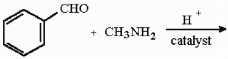

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
Pyridine does not undergo Friedel-Crafts reactions. Offer an explanation.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
Provide the structure of the major organic product in the reaction below. 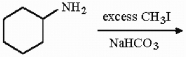
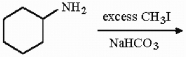

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Can 1-hexanamine be prepared in good yield from the reaction of a 1:1 molar ratio of ammonia and 1-bromohexane? Why or why not?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is an intermediate in the mechanism for amide synthesis through acylation of an amine? 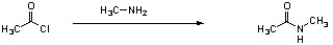
A)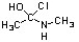
B)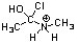
C)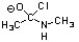
D)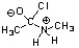

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
Which compound would react most rapidly with sodium methoxide and heat?
A) pyridine
B) 2-chloropyridine
C) 3-chloropyridine
D) 4-chloropyridine
A) pyridine
B) 2-chloropyridine
C) 3-chloropyridine
D) 4-chloropyridine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
What reagent is needed to accomplish the transformation below? 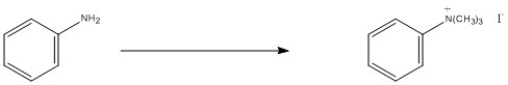


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is ________.
A) 2-nitropyridine
B) 3-nitropyridine
C) 4-nitropyridine
D) 3-aminopyridine
E) 4-aminopyridine
A) 2-nitropyridine
B) 3-nitropyridine
C) 4-nitropyridine
D) 3-aminopyridine
E) 4-aminopyridine

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
Predict the major organic product of the following reaction: 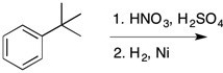


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
Can 2-methyl-2-butanamine be prepared in good yield from the reaction of ammonia with 2-bromo-2-methylbutane? Why or why not?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the major organic product of the following reaction. 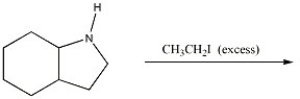


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following chloropyridines readily undergo nucleophilic substitution upon treatment with NaCN?
A) 2-chloropyridine
B) 3-chloropyridine
C) 4-chloropyridine
D) both A and C
E) both A and B
A) 2-chloropyridine
B) 3-chloropyridine
C) 4-chloropyridine
D) both A and C
E) both A and B

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


