Exam 19: Amines
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
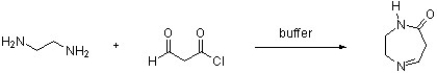
Draw a structure for the expected product of the following reaction. 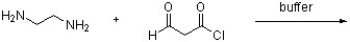
Free
(Essay)
4.8/5  (43)
(43)
Correct Answer:
Which of the following compounds is a 2° amine?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
C
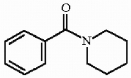
Provide the structure of the major organic product in the reaction below. 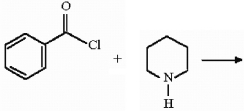
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
Compound A, C9H17N, is an optically inactive alkaloid, containing a tertiary amine that is not located at the bridgehead of the structures fused bicyclic ring system. Exhaustive alkylation of A with methyl iodide, followed by a Hofmann elimination reaction resulted in a racemic mixture of chiral tertiary amine products B, C10H19N. Provide a structure for A and one of the enantiomeric products B.
(Essay)
4.8/5  (28)
(28)
Can 1-hexanamine be prepared in good yield from the reaction of a 1:1 molar ratio of ammonia and 1-bromohexane? Why or why not?
(Essay)
4.9/5  (32)
(32)
Provide the structure of the major organic product in the reaction below. 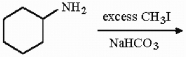
(Essay)
4.9/5  (36)
(36)
What sequence of reagents can be used to carry out the transformation shown below? 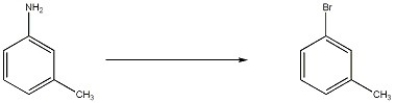
(Short Answer)
4.8/5  (32)
(32)
The nitrogen's lone pair in pyrrolidine is best described as occupying what type of orbital?
(Multiple Choice)
4.9/5  (28)
(28)
When an arenediazonium ion reacts with an activated aromatic ring, the product is ________.
(Multiple Choice)
4.9/5  (40)
(40)
The product of the reaction below has been found to be a potent anticancer agent (J. Med. Chem. 2010, 6867). Predict the structure of the product of the following reaction. 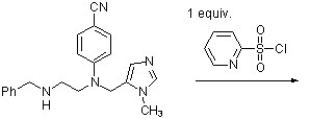
(Essay)
4.7/5  (35)
(35)
The following structure has been used in monitoring the development of amyloid plaques in Alzheimer's patients (J. Med. Chem. 2011, 949). Which sequence correctly ranks the following nitrogens in order of increasing pKb value? 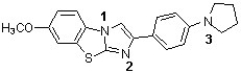
(Multiple Choice)
4.9/5  (35)
(35)
Secondary amines react with the nitrosonium ion to generate ________.
(Multiple Choice)
4.9/5  (38)
(38)
Predict the product of the reaction shown below and add arrows to show the mechanism. 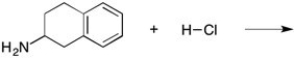
(Essay)
4.8/5  (31)
(31)
Can 2-methyl-2-butanamine be prepared in good yield from the reaction of ammonia with 2-bromo-2-methylbutane? Why or why not?
(Essay)
4.8/5  (31)
(31)
Provide the structure of the major organic product in the reaction below. 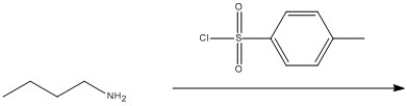
(Essay)
4.8/5  (33)
(33)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)