Deck 2: Acids and Bases; Functional Groups
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/136
Play
Full screen (f)
Deck 2: Acids and Bases; Functional Groups
1
The electron density at any point is proportional to the ________ of the electron wave at that point.
square of the wave function
2
How many π bonds are present in the molecule shown? 
A) 0
B) 1
C) 2
D) 4
E) 6

A) 0
B) 1
C) 2
D) 4
E) 6
2
3
How many π bonds are present in the molecule shown? 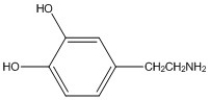
A) 0
B) 1
C) 3
D) 4
E) 6

A) 0
B) 1
C) 3
D) 4
E) 6
3
4
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 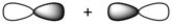
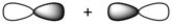

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the interaction of two hydrogen 1s atomic orbitals of the same phase. Which of the statements below is an incorrect description of this interaction?
A) A sigma bonding molecular orbital is formed.
B) The molecular orbital formed is lower in energy than a hydrogen 1s atomic orbital.
C) The molecular orbital formed has a node between the atoms.
D) The molecular orbital formed is cylindrically symmetric.
E) A maximum of two electrons may occupy the molecular orbital formed.
A) A sigma bonding molecular orbital is formed.
B) The molecular orbital formed is lower in energy than a hydrogen 1s atomic orbital.
C) The molecular orbital formed has a node between the atoms.
D) The molecular orbital formed is cylindrically symmetric.
E) A maximum of two electrons may occupy the molecular orbital formed.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
6
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 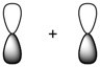


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
7
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 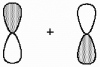


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
8
When orbitals on different atoms interact, ________ are produced.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
9
An orbital can be described by its ________, which is the mathematical description of the shape of the electron wave as it oscillates.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
10
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 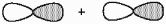
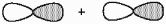

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
11
How many carbon-carbon σ bonds are present in the molecule shown? 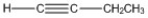
A) 1
B) 2
C) 3
D) 4
E) 5
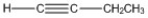
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
12
Two p orbitals can overlap to form a σ molecular orbital. How many nodes are present in this σ molecular orbital?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
13
How many carbon-carbon σ bonds are present in the molecule shown? 
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
14
A ________ bond results when parallel p orbitals overlap sideways.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
15
How many carbon-carbon σ bonds are present in the molecule shown? 
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
16
If a compound, C5H7NO, contains 1 ring, how many pi bonds are there in this compound?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
17
Two p orbitals can overlap to form a σ* molecular orbital. How many nodes are present in this σ* molecular orbital?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements about π molecular orbitals is/are correct?
A) π molecular orbitals are cylindrically symmetric.
B) Most of the electron density in a π molecular orbital is centered above and below the internuclear axis.
C) When two atoms are connected by a double bond, both of these bonds are π bonds.
D) Both statements B and C are correct.
E) Statements A, B, and C are all correct.
A) π molecular orbitals are cylindrically symmetric.
B) Most of the electron density in a π molecular orbital is centered above and below the internuclear axis.
C) When two atoms are connected by a double bond, both of these bonds are π bonds.
D) Both statements B and C are correct.
E) Statements A, B, and C are all correct.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
19
Which atomic orbital combination would result in a molecular sigma bond?
A)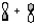
B)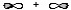
C)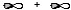
D)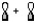
A)
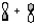
B)
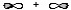
C)
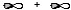
D)
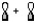

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
20
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 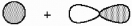
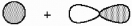

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
21
What two hybrid atomic orbitals overlap to form the C-C σ bond in acetonitrile, CH3C≡N?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
22
Shown below is one of the sex pheremones from the butterfly family. How many sp2 hybridized carbon atoms are present in this molecule? 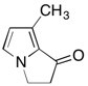
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
23
In the structure below, the hybridization of the oxygen is ________ and the C-O-C bond angle is ________. 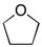
A) sp2; 120°
B) sp2; <109.5°
C) sp3; 120°
D) sp3; <109.5°
E) sp; 120°

A) sp2; 120°
B) sp2; <109.5°
C) sp3; 120°
D) sp3; <109.5°
E) sp; 120°

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
24
Draw a three-dimensional model of methanol, CH3OH, including lone pairs of electrons using wedges, dashes and straight lines.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the term below which best describes the geometry of acetylene (HCCH).
A) trigonal bipyramidal
B) trigonal
C) tetrahedral
D) square planar
E) linear
A) trigonal bipyramidal
B) trigonal
C) tetrahedral
D) square planar
E) linear

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
26
Choose the correct hybridization for the atom indicated in the molecule below.
CH3CH2CH2CH3
↑
A) sp
B) sp2
C) sp3
D) none of the above
CH3CH2CH2CH3
↑
A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
27
In the structure below, the sigma bond of the carbonyl is formed from the overlap of a(n) ________ atomic orbital of carbon and a(n) ________ atomic orbital of oxygen. 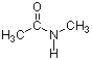
A) sp, sp2
B) sp3, sp2
C) sp2, sp2
D) p, p

A) sp, sp2
B) sp3, sp2
C) sp2, sp2
D) p, p

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
28
What is the approximate value of the CCC bond angle in CH3CH2CH2OH?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
29
What two hybrid atomic orbitals overlap to form the C-C σ bond in allene, H2C=C=CH2?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the correct hybridization for the atom indicated in the molecule below.
CH3CH2CH2CH3
↑
A) sp
B) sp2
C) sp3
D) none of the above
CH3CH2CH2CH3
↑
A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
31
The HCH bond angle in allene (H2CCCH2) is ________.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
32
Vildagliptin is a recently released antidiabetic drug (J. Med. Chem. 2010, 7902). How many elements of unsaturation are in Vildagliptin? 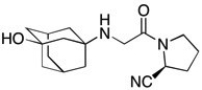
A) 4
B) 5
C) 6
D) 7

A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
33
Shown below is one of the sex pheremones from the butterfly family. How many sp3 hybridized carbon atoms are present? 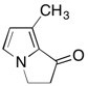
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
34
What is the approximate value of the CCC bond angle in CH3C≡CCH3?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
35
Circle the coplanar atoms in 1-ethylcyclopentene shown below. 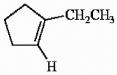


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
36
Based on the structure below, what is the value for the H-N-CH3 bond angle? 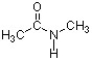
A) 60 degrees
B) 90 degrees
C) 109 degrees
D) 120 degrees

A) 60 degrees
B) 90 degrees
C) 109 degrees
D) 120 degrees

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
37
Complete the structure of methyl azide by adding any necessary formal charges. 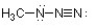
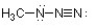

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
38
What is the approximate value of any HCC bond angle in H2C=CHCCl3?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
39
Shown below is one of the sex pheremones from the butterfly family. How many sp hybridized carbon atoms are present in this molecule? 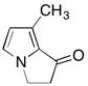
A) 0
B) 1
C) 2
D) 3
E) 5

A) 0
B) 1
C) 2
D) 3
E) 5

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
40
What two hybrid atomic orbitals overlap to form the C-C σ bond in acetaldehyde, CH3CHO?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
41
The CCN bond angle in acrylonitrile (CH2=CHCN) is approximately ________.
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the correct hybridization for the atom indicated in the molecule below.
(CH3)2CHCN
↑
A) sp
B) sp2
C) sp3
D) none of the above
(CH3)2CHCN
↑
A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
43
Acrylonitrile (CH2=CHCN) contains ________ σ bonds and ________ π bonds.
A) 4; 1
B) 6; 3
C) 4; 3
D) 6; 1
E) 2; 0
A) 4; 1
B) 6; 3
C) 4; 3
D) 6; 1
E) 2; 0

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the correct hybridization for the atom indicated in the molecule below.
(CH3)2CHCN
↑
A) sp
B) sp2
C) sp3
D) none of the above
(CH3)2CHCN
↑
A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
45
Choose the correct hybridization for the atom indicated in the molecule below. 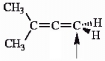
A) sp
B) sp2
C) sp3
D) none of the above

A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
46
How many σ bonds does the compound shown contain? 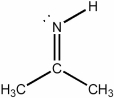
A) 3
B) 5
C) 9
D) 10
E) 11

A) 3
B) 5
C) 9
D) 10
E) 11

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
47
There is one more important resonance form for the structure below. Draw it and then indicate the hybridization of all of the non-H atoms. 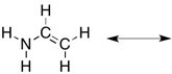


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
48
The CCO bond angle in acetone (CH3COCH3) is ________.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
49
What is the hybridization of the nitrogen atom in the molecule below? 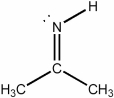
A) s
B) sp
C) sp2
D) sp3
E) none of the above

A) s
B) sp
C) sp2
D) sp3
E) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
50
What is the approximate CCC bond angle in the compound below? 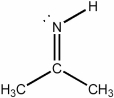
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements concerning the cyclic molecule shown is not true? 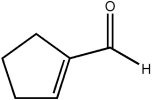
A) It contains a π molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen p atomic orbital.
B) It contains a σ molecular orbital formed by the overlap of two carbon sp2 hybrid atomic orbitals.
C) It contains a σ molecular orbital formed by the overlap of two carbon sp3 hybrid atomic orbitals.
D) It contains a π molecular orbital formed by the overlap of two carbon p atomic orbitals.
E) It contains a σ molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen sp3 atomic orbital.

A) It contains a π molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen p atomic orbital.
B) It contains a σ molecular orbital formed by the overlap of two carbon sp2 hybrid atomic orbitals.
C) It contains a σ molecular orbital formed by the overlap of two carbon sp3 hybrid atomic orbitals.
D) It contains a π molecular orbital formed by the overlap of two carbon p atomic orbitals.
E) It contains a σ molecular orbital formed by the overlap of a carbon p atomic orbital with an oxygen sp3 atomic orbital.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
52
The structure of uracil is shown below. What is the hybridization of the nitrogens? 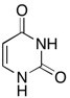


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
53
The molecule shown below contains ________ pi bonds and ________ sigma bonds. 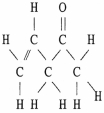


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
54
The HNC bond angle in the cation [CH2NH2]+ is approximately ________.
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the labeled atoms in the following structure are sp2 hybridized? 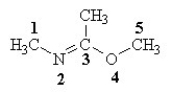
A) 1 & 4
B) 2 & 5
C) 2 & 4
D) 2 & 3
E) 2, 3, & 4

A) 1 & 4
B) 2 & 5
C) 2 & 4
D) 2 & 3
E) 2, 3, & 4

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
56
Triethylamine [(CH3CH2)3N] is a molecule in which the nitrogen atom is ________ hybridized and the CNC bond angle is ________.
A) sp2; >109.5°
B) sp2; <109.5°
C) sp3; >109.5°
D) sp3; <109.5°
E) sp; 109.5°
A) sp2; >109.5°
B) sp2; <109.5°
C) sp3; >109.5°
D) sp3; <109.5°
E) sp; 109.5°

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
57
Choose the correct hybridization for the atom indicated in the molecule below. 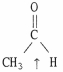
A) sp
B) sp2
C) sp3
D) none of the above

A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
58
The structure of uracil is shown below. What is the molecular shape of the nitrogens? 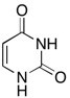


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
59
The HCN bond angle in hydrogen cyanide (HCN) is ________.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
60
The hybridization of the nitrogen in the molecule shown below is sp2. Briefly explain why. 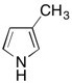


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
61
Choose the correct hybridization for the atom indicated in the molecule below. 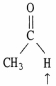
A) sp
B) sp2
C) sp3
D) none of the above

A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following compounds is not a constitutional isomer of a compound with an empirical formula C3H7O and a formula mass of 118.164?
A)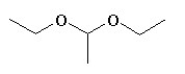
B)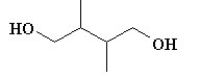
C)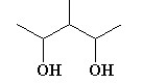
D)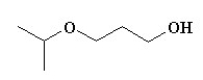
E)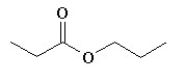
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the hybridization of oxygen in acetaldehyde (CH3CHO) and estimate the OCH bond angle.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
64
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
65
Boron trifluoride (BF3) is a molecule in which the boron atom is ________ hybridized and the FBF bond angle is ________.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
66
Structures which differ only in rotations about a single bond are called ________.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
67
Explain why the free rotation about the carbon-carbon bond in CH3CH3 is not present in 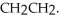
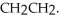

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
68
From a molecular orbital perspective, why is there relatively free rotation about the carbon-carbon bond of ethane (CH3CH3)?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
69
What hybrid atomic orbitals are overlapping to form the carbon-oxygen σ bond in acetaldehyde (CH3CHO)?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the skeletal structures of the five constitutional isomers with molecular formula C6H14.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
71
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 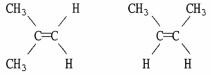
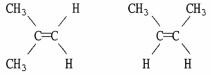

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
72
The molecule shown below contains ________ sigma bonds and ________ pi bonds. 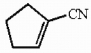


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
73
Choose the correct hybridization for the atom indicated in the molecule below. 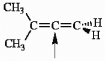
A) sp
B) sp2
C) sp3
D) none of the above

A) sp
B) sp2
C) sp3
D) none of the above

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
74
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
75
From a molecular orbital perspective why isn't there relatively free rotation about the carbon-carbon double bond in ethene (CH2=CH2)?

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following best describes the relationship between the two structures shown? 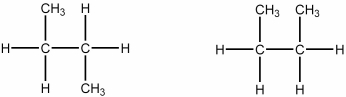
A) They represent the same compound.
B) They represent different compounds that are constitutional isomers.
C) They represent different compounds that are geometric isomers.
D) They represent different compounds that are alkenes.
E) They represent different compounds that are alkanes.
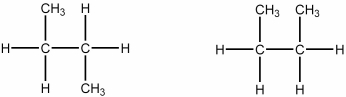
A) They represent the same compound.
B) They represent different compounds that are constitutional isomers.
C) They represent different compounds that are geometric isomers.
D) They represent different compounds that are alkenes.
E) They represent different compounds that are alkanes.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
77
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 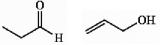
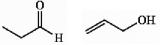

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
78
Provide the hybridization of oxygen in dimethyl ether (CH3OCH3) and estimate the COC bond angle.

Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
79
The synthetic steroid RU-486 is shown below. How many pi bonds does RU-486 contain? 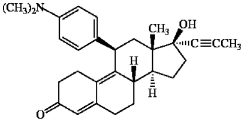


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck
80
Are the two compounds shown below best described as cis-trans isomers, constitutional isomers, or not isomeric? 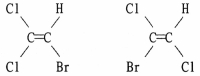


Unlock Deck
Unlock for access to all 136 flashcards in this deck.
Unlock Deck
k this deck


