Exam 2: Acids and Bases; Functional Groups
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
What intermolecular attractions exist in a pure sample of methylthiol, CH3SH?
Free
(Essay)
4.7/5  (34)
(34)
Correct Answer:
London dispersion forces and dipole-dipole attractions.
The hybridization of the nitrogen in the molecule shown below is sp2. Briefly explain why. 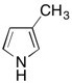
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
This molecule has resonance structures where the lone pair on the nitrogen is delocalized in to the ring through a pi bond. This requires the nitrogen to be sp2 hybridized. 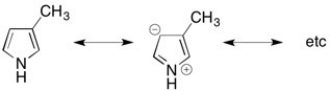
Use the following structure for the two questions below.
Saquinavir Structure 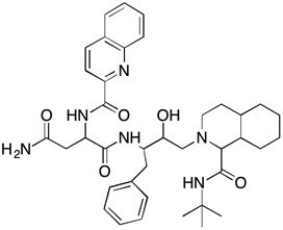 -Which of the molecules below can be properly called an amine?
-Which of the molecules below can be properly called an amine?
(Multiple Choice)
4.8/5  (38)
(38)
What kind of molecular orbital (σ, σ*, π, or π*) results when the two atomic orbitals shown below interact in the manner indicated? 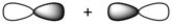
(Not Answered)
This question doesn't have any answer yet
The HNC bond angle in the cation [CH2NH2]+ is approximately ________.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following statements about π molecular orbitals is/are correct?
(Multiple Choice)
4.9/5  (34)
(34)
Sodium hydride (NaH) is a base that is commonly used in organic reactions. The pKa of H2 is 36. Which of the following compounds could not be used as a solvent if you were using NaH?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements concerning the cyclic molecule shown is not true? 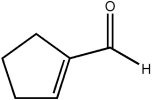
(Multiple Choice)
4.7/5  (40)
(40)
An orbital can be described by its ________, which is the mathematical description of the shape of the electron wave as it oscillates.
(Short Answer)
4.9/5  (27)
(27)
Choose the correct hybridization for the atom indicated in the molecule below. 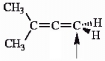
(Multiple Choice)
4.9/5  (34)
(34)
Consider 1,2-dibromoethene, shown below. Use arrows to represent the individual bond dipoles. Would you expect this molecule to be polar? Briefly explain your reasoning. 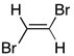
(Essay)
4.9/5  (33)
(33)
How many carbon-carbon σ bonds are present in the molecule shown? 
(Multiple Choice)
4.8/5  (34)
(34)
Clonazapam (TM) is used to treat seizures and panic disorders and is shown below. Are there any aromatic hydrocarbons in the molecule? If so, circle them. 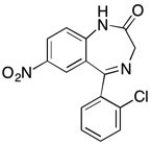
(Essay)
4.7/5  (26)
(26)
Draw a three-dimensional model of methanol, CH3OH, including lone pairs of electrons using wedges, dashes and straight lines.
(Essay)
4.9/5  (36)
(36)
Choose the correct hybridization for the atom indicated in the molecule below. 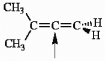
(Multiple Choice)
4.8/5  (41)
(41)
From a molecular orbital perspective why isn't there relatively free rotation about the carbon-carbon double bond in ethene (CH2=CH2)?
(Essay)
4.8/5  (35)
(35)
Choose the correct hybridization for the atom indicated in the molecule below. 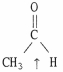
(Multiple Choice)
4.7/5  (38)
(38)
Vildagliptin is a recently released antidiabetic drug (J. Med. Chem. 2010, 7902). Circle and name each functional group in vildagliptin. 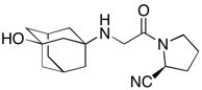
(Essay)
4.9/5  (38)
(38)
Showing 1 - 20 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)