Deck 23: Carbohydrates and Nucleic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/126
Play
Full screen (f)
Deck 23: Carbohydrates and Nucleic Acids
1
L-Ribulose may be classified as ________.
A) an aldotetrose
B) an aldopentose
C) an aldohexose
D) a ketotetrose
E) a ketopentose
A) an aldotetrose
B) an aldopentose
C) an aldohexose
D) a ketotetrose
E) a ketopentose
a ketopentose
2
Draw the Fischer projection for the open-chain form of D-glucose.
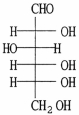
3
Draw the Fischer projection of the open chain form of L-allose.
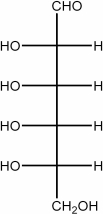
4
Draw the structure of D-glyceraldehyde.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
5
Given the structure of D-altrose below, draw the structure of L-altrose. 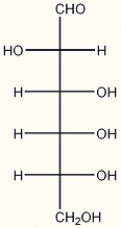


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
6
Draw the Fischer projection for the open-chain form of D-erythrose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
7
The structure of D-arabinose is shown below. Which of the following correctly describes the configurations of the asymmetric carbons in D-arabinose? 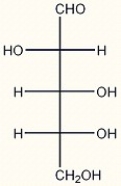
A) 2S, 3R, 4R
B) 2R, 3S, 4S
C) 2S, 3R, 4S
D) 2R, 3S, 4R
E) 2S, 3S, 4R

A) 2S, 3R, 4R
B) 2R, 3S, 4S
C) 2S, 3R, 4S
D) 2R, 3S, 4R
E) 2S, 3S, 4R

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
8
Most monosaccharides are highly water soluble. Offer an explanation.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
9
Draw the cyclic hemiacetal form of D-glucose both as a chair conformation and as a Haworth projection.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the C2 epimer of D-glucose?
A)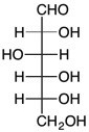
B)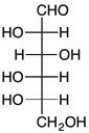
C)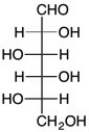
D)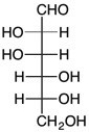
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
11
Draw the Fischer projection of the open chain form of D-arabinose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
12
How many asymmetric centers are present in the open chain form of the aldohexose D-(-)-gulose?
A) 0
B) 1
C) 2
D) 4
E) 5
A) 0
B) 1
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
13
All naturally occurring sugars can be degraded to ________.
A) D-(-)-glyceraldehyde
B) D-(+)-glyceraldehyde
C) L-(-)-glyceraldehyde
D) L-(+)-glyceraldehyde
E) none of the above
A) D-(-)-glyceraldehyde
B) D-(+)-glyceraldehyde
C) L-(-)-glyceraldehyde
D) L-(+)-glyceraldehyde
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
14
Draw the Fischer projection for the open-chain form of D-fructose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
15
All chiral D-sugars rotate plane-polarized light ________.
A) clockwise
B) counterclockwise
C) +20.0°
D) in a direction that cannot be predicted but must be determined experimentally
E) since they are optically inactive
A) clockwise
B) counterclockwise
C) +20.0°
D) in a direction that cannot be predicted but must be determined experimentally
E) since they are optically inactive

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
16
How many molecules of carbon dioxide and water are needed to make a molecule of glucose in photosynthesis?
A) 5 CO2 and 5 H2O
B) 5 CO2 and 6 H2O
C) 6 CO2 and 6 H2O
D) 6 CO2 and 2 H2O
E) 2 CO2 and 2 H2O
A) 5 CO2 and 5 H2O
B) 5 CO2 and 6 H2O
C) 6 CO2 and 6 H2O
D) 6 CO2 and 2 H2O
E) 2 CO2 and 2 H2O

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
17
Draw the C3 epimer of L-glucose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
18
In the Fischer projection of D-(+)-glyceraldehyde, the hydroxyl group on the asymmetric carbon center is ________.
A) on the bottom
B) on the top
C) at the left
D) at the right
E) present as a hemiacetal
A) on the bottom
B) on the top
C) at the left
D) at the right
E) present as a hemiacetal

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
19
Provide the structure of the open-chain form of D-galactose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
20
Provide the structure of the open-chain form of L-altrose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following correctly describes the structural relationship between D-gulose and L-gulose?
A) diastereomers and epimers
B) diastereomers but not epimers
C) enantiomers
D) constitutional isomers
E) not isomers
A) diastereomers and epimers
B) diastereomers but not epimers
C) enantiomers
D) constitutional isomers
E) not isomers

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the C3 epimer of the sugar below drawn in its open chain (acyclic) Fischer projection. 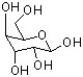
A)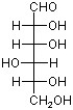
B)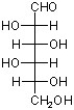
C)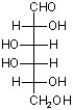
D)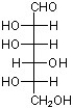

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the Fischer projection of (2S,3R)-2,3-dihydroxybutanoic acid, and label it as erythro or threo.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following descriptions apply to both D-glucopyranose and L-galactopyranose?
I. They are both ketoses
II. They are both aldoses
III. They are enantiomers of each other
IV. They are diastereomers of each other
V. They are epimers of each other
A) I, III and V
B) II and III
C) I, IV and V
D) II and IV
E) II, IV and V
I. They are both ketoses
II. They are both aldoses
III. They are enantiomers of each other
IV. They are diastereomers of each other
V. They are epimers of each other
A) I, III and V
B) II and III
C) I, IV and V
D) II and IV
E) II, IV and V

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
25
Fructose forms ________.
A) a five-membered cyclic hemiacetal
B) a six-membered cyclic hemiacetal
C) a six-membered lactone
D) a five-membered lactone
E) none of the above
A) a five-membered cyclic hemiacetal
B) a six-membered cyclic hemiacetal
C) a six-membered lactone
D) a five-membered lactone
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following correctly describes the structural relationship between D-ribose and L-lyxose?
A) diastereomers and epimers
B) diastereomers but not epimers
C) enantiomers
D) constitutional isomers
E) not isomers
A) diastereomers and epimers
B) diastereomers but not epimers
C) enantiomers
D) constitutional isomers
E) not isomers

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following descriptions apply to both dihydroxyacetone and D-fructofuranose?
I. They are both ketoses.
II. They are both aldoses.
III. They are enatiomers of each other.
IV. They are diastereomers of each other.
V. They are epimers of each other.
A) I
B) II
C) I and III
D) II and IV
E) II, IV and V
I. They are both ketoses.
II. They are both aldoses.
III. They are enatiomers of each other.
IV. They are diastereomers of each other.
V. They are epimers of each other.
A) I
B) II
C) I and III
D) II and IV
E) II, IV and V

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the Haworth structure of β-D-ribofuranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
29
Stereoisomeric aldohexoses that differ in configuration at only a single carbon are ________.
A) meso compounds
B) threo sugars
C) enantiomers
D) constitutional isomers
E) epimers
A) meso compounds
B) threo sugars
C) enantiomers
D) constitutional isomers
E) epimers

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
30
How many asymmetric carbons are present in α-D-ribopyranose?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
31
In a Haworth projection of a five-membered cyclic hemiacetal, ________.
A) the C6 carbon is drawn on the right
B) the C2 carbon is drawn on the left
C) the ring oxygen is drawn in the front
D) the ring oxygen is drawn in the back
E) all hydroxyls are always on the same side of the molecule
A) the C6 carbon is drawn on the right
B) the C2 carbon is drawn on the left
C) the ring oxygen is drawn in the front
D) the ring oxygen is drawn in the back
E) all hydroxyls are always on the same side of the molecule

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the Haworth structure of β-D-glucopyranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the Haworth structure of α-D-ribofuranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following represents a pair of epimers?
A) D- and L-erythrose
B) D-erythrose and D-threose
C) D-arabinose and D-erythrose
D) D-arabinose and D-xylose
E) D-glyceraldehyde and D-threose
A) D- and L-erythrose
B) D-erythrose and D-threose
C) D-arabinose and D-erythrose
D) D-arabinose and D-xylose
E) D-glyceraldehyde and D-threose

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the Haworth structure of α-D-glucopyranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the more stable chair conformer of α-D-glucopyranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
37
When a monosaccharide reacts to give the pyranose form from its open-chain form, how many distinct pyranose forms are possible?
A) 1
B) 2
C) 2n, where n is the number of carbons present
D) 4n + 2, where n is the number of carbons present
E) 4
A) 1
B) 2
C) 2n, where n is the number of carbons present
D) 4n + 2, where n is the number of carbons present
E) 4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
38
Six-membered cyclic hemiacetals and five-membered cyclic hemiacetals are called, respectively, ________.
A) mannoses and xyloses
B) maltoses and arabinoses
C) pyranoses and furanoses
D) glyoses and fructoses
E) none of the above
A) mannoses and xyloses
B) maltoses and arabinoses
C) pyranoses and furanoses
D) glyoses and fructoses
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
39
In solution, glucose exists as ________.
A) the open-chain form only
B) the cyclic hemiacetal form only
C) an equilibrium mixture of the open-chain form and cyclic acetal forms
D) an equilibrium mixture of the open-chain form and cyclic hemiacetal forms
A) the open-chain form only
B) the cyclic hemiacetal form only
C) an equilibrium mixture of the open-chain form and cyclic acetal forms
D) an equilibrium mixture of the open-chain form and cyclic hemiacetal forms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
40
A diastereomer is called erythro if its Fischer projection shows similar groups ________.
A) opposite each other on the same carbon
B) equally spaced about a central carbonyl
C) directly above and below a central carbonyl
D) on the same side of the molecule on adjacent carbons
E) on opposite sides of the molecule on adjacent carbons
A) opposite each other on the same carbon
B) equally spaced about a central carbonyl
C) directly above and below a central carbonyl
D) on the same side of the molecule on adjacent carbons
E) on opposite sides of the molecule on adjacent carbons

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the Haworth structure of the α-pyranose form of the monosaccharide shown below. 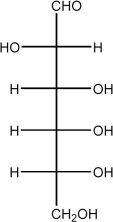


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the Haworth structure of the α-furanose form of the monosaccharide shown below. 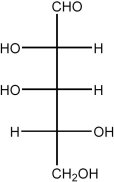


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
43
What reagents are needed to induce the following isomerization? 
A) NaOH
B) Br2 / H2O
C) HCl (aq)
D) PCC

A) NaOH
B) Br2 / H2O
C) HCl (aq)
D) PCC

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
44
Reduction of a 2-ketohexose with NaBH4 yields ________.
A) a single aldohexose
B) a mixture of acetals
C) a mixture of alditols
D) a mixture of cyclic hemiacetals
E) a single pyranose
A) a single aldohexose
B) a mixture of acetals
C) a mixture of alditols
D) a mixture of cyclic hemiacetals
E) a single pyranose

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
45
When L-erythrose is treated with NaBH4, ________.
A) a 70:30 mixture of enantiomeric alditols results
B) a 50:50 mixture of enantiomeric alditols results
C) a meso alditol is produced
D) the product mixture contains two diastereomeric alditols
E) an optically active alditol is produced
A) a 70:30 mixture of enantiomeric alditols results
B) a 50:50 mixture of enantiomeric alditols results
C) a meso alditol is produced
D) the product mixture contains two diastereomeric alditols
E) an optically active alditol is produced

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
46
Anomers of D-glucopyranose differ in their stereochemistry at ________.
A) C5
B) C4
C) C3
D) C2
E) C1
A) C5
B) C4
C) C3
D) C2
E) C1

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the Haworth structure of the α-pyranose form of the monosaccharide shown below. 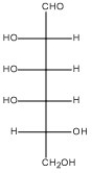


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the Haworth structure of the β-furanose form of the monosaccharide shown below. 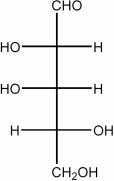


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
49
How might one separate α-D-glucopyranose from a solution also containing 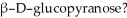
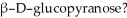

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
50
The α and β anomers of a D-glucopyranose are related as ________.
A) enantiomers
B) meso compounds
C) structural isomers
D) epimers
E) disaccharides
A) enantiomers
B) meso compounds
C) structural isomers
D) epimers
E) disaccharides

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
51
When pure α-D-glucopyranose is dissolved in water, the optical rotation of the resulting solution changes over a period of time. What is the name of this phenomenon and why does it occur?

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
52
Show the Haworth of α-L-fructofuranose and the Fischer diagram of L-fructose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the product of the following reaction in a Fischer projection. 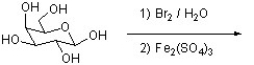


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the Haworth structure of the α-pyranose form of the monosaccharide shown below. 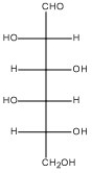


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
55
What are the two most common unwanted side reactions which can occur when monosaccharides are treated with base?
A) epimerization and enediol rearrangement
B) mutarotation and epimerization
C) enediol rearrangement and glycoside formation
D) Kiliani-Fischer and Ruff degradations
E) Ruff degradation and glycoside formation
A) epimerization and enediol rearrangement
B) mutarotation and epimerization
C) enediol rearrangement and glycoside formation
D) Kiliani-Fischer and Ruff degradations
E) Ruff degradation and glycoside formation

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
56
What is the result of reaction involving D-glyceraldehyde and NaBH4?
A) propane
B) ethanol and CO2
C) dihydoxyacetone
D) glyceric acid
E) glycerol
A) propane
B) ethanol and CO2
C) dihydoxyacetone
D) glyceric acid
E) glycerol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the Haworth structure of β-D-ribopyranose.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
58
Draw the Haworth structure of the α-furanose form of the monosaccharide shown below. 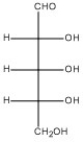


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the Haworth structure of the β-pyranose form of the monosaccharide shown below. 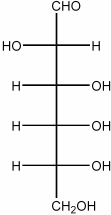


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
60
A pyranose with the hydroxyl group on the anomeric carbon pointing up in the Haworth structure is designated ________.
A) a'
B) b'
C) α
D) β
E) γ
A) a'
B) b'
C) α
D) β
E) γ

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
61
When L-(+)-idose is treated with bromine water, which of the following results?
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
62
What is the major organic product of the following reaction? 


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
63
What is the major organic product of the following reaction? 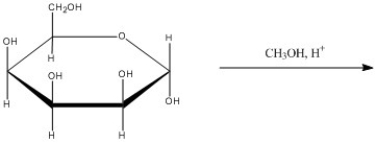


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
64
When D-(+)-allose is treated with bromine water, which of the following results?
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
65
When D-(+)-xylose is treated with nitric acid, which of the following results?
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
66
Provide the structure of the product which results when D-ribose is treated with bromine water.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is true?
A) All monosaccharides in either the ketose of aldose family are reducing sugars.
B) Only aldoses, but not ketoses, are reducing sugars.
C) Only ketoses, but not aldoses, are reducing sugars.
D) All disaccharides, not monosaccharides, are reducing sugars.
E) All glycosides are reducing sugars.
A) All monosaccharides in either the ketose of aldose family are reducing sugars.
B) Only aldoses, but not ketoses, are reducing sugars.
C) Only ketoses, but not aldoses, are reducing sugars.
D) All disaccharides, not monosaccharides, are reducing sugars.
E) All glycosides are reducing sugars.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
68
What is the major organic product of the following reaction? 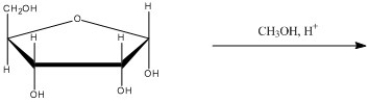


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
69
What is the outcome of the following reaction? 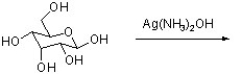
A) No Reaction
B)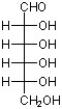
C)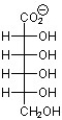
D)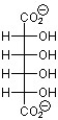

A) No Reaction
B)

C)

D)


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
70
Under what conditions is the methyl glycoside of galactose prepared?

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
71
Draw the Haworth structure of methyl α-D-xylofuranoside.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following would give a positive Tollen's test?
A) α-D-glucopyranose
B) methyl β-D-glucopyranoside
C) sucrose
D) methyl α-D-ribofuranoside
E) none of the above
A) α-D-glucopyranose
B) methyl β-D-glucopyranoside
C) sucrose
D) methyl α-D-ribofuranoside
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
73
When L-(+)-lyxose is treated with nitric acid, which of the following results?
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid
A) a racemic mixture of aldaric acids
B) an optically active aldaric acid
C) an optically inactive aldaric acid
D) an optically active aldonic acid
E) an optically inactive aldonic acid

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the Haworth structure of ethyl β-D-galactopyranoside.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
75
Under what conditions is the methyl glycoside of galactose hydrolyzed?

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
76
Provide the Fischer projection of the open-chain form of the aldonic acid which results when L-glucose is treated with bromine water.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is a reducing sugar?
A) α-D-fructofuranose
B) the methyl glycoside of cellobiose
C) ethyl β-D-glucopyranoside
D) methyl α-D-fructofuranoside
E) none of the above
A) α-D-fructofuranose
B) the methyl glycoside of cellobiose
C) ethyl β-D-glucopyranoside
D) methyl α-D-fructofuranoside
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
78
Will methyl-β-D-talopyranoside undergo mutarotation? Why or why not?

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
79
The open-chain form of D-talose is shown below. Draw the chair form of methyl β-D-talopyranoside. 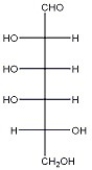


Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
80
An aglycone is a group bound to ________.
A) the oxygen of C6 in a glycoside
B) the anomeric carbon in a glycoside
C) a pyrimidine base
D) a purine base
E) a xanthate
A) the oxygen of C6 in a glycoside
B) the anomeric carbon in a glycoside
C) a pyrimidine base
D) a purine base
E) a xanthate

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck


