Exam 23: Carbohydrates and Nucleic Acids
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
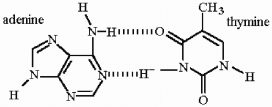
Show the hydrogen bonding which occurs when adenine and thymine form a base pair.
Free
(Essay)
4.8/5  (39)
(39)
Correct Answer:
When L-erythrose is treated with NaBH4, ________.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
C
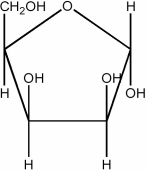
Draw the Haworth structure of the α-furanose form of the monosaccharide shown below. 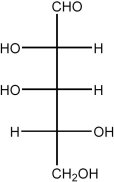
Free
(Essay)
4.8/5  (29)
(29)
Correct Answer:
Draw the Haworth structure of the β-pyranose form of the monosaccharide shown below. 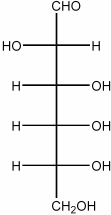
(Essay)
4.7/5  (46)
(46)
When D-(+)-allose is treated with bromine water, which of the following results?
(Multiple Choice)
4.9/5  (32)
(32)
The structure of D-arabinose is shown below. Which of the following correctly describes the configurations of the asymmetric carbons in D-arabinose? 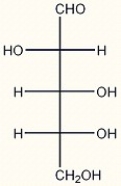
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following descriptions apply to both dihydroxyacetone and D-fructofuranose?
I. They are both ketoses.
II. They are both aldoses.
III. They are enatiomers of each other.
IV. They are diastereomers of each other.
V. They are epimers of each other.
(Multiple Choice)
4.8/5  (30)
(30)
Two D-aldopentoses give the same D-aldotetrose upon Ruff degradation. The two aldopentoses are ________.
(Multiple Choice)
4.8/5  (42)
(42)
Draw the Haworth structure of the α-pyranose form of the monosaccharide shown below. 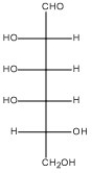
(Essay)
4.8/5  (44)
(44)
Which of the following nitrogens of adenine connects to ribose to form a nucleoside? 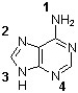
(Multiple Choice)
4.7/5  (29)
(29)
Identify the C3 epimer of the sugar below drawn in its open chain (acyclic) Fischer projection. 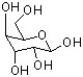
(Multiple Choice)
4.8/5  (31)
(31)
Draw the Haworth structure of the α-furanose form of the monosaccharide shown below. 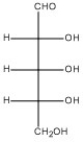
(Essay)
4.9/5  (23)
(23)
The open-chain form of D-talose is shown below. Draw the chair form of methyl β-D-talopyranoside. 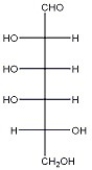
(Essay)
4.9/5  (32)
(32)
Provide the structure of the major organic product which results when D-ribose is subjected to a Ruff degradation.
(Essay)
4.7/5  (34)
(34)
D-Tagatose (below) is a naturally occurring sweetener that is 92% as sweet as sucrose but has only 38% of the calories. Which epimer of D-glucose undergoes keto-enol tautomerization to give D-tagatose? 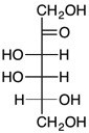
(Short Answer)
4.9/5  (40)
(40)
Showing 1 - 20 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)