Deck 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
1
Draw the most stable conformation of (2Z,4E)-2,4-hexadiene.
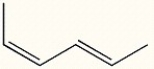
2
Rank the following dienes in order of increasing stability: trans-1,3-pentadiene, cis-1,3-pentadiene, 1,4-pentadiene, and 1,2-pentadiene.
1,2-pentadiene < 1,4-pentadiene < cis-1,3-pentadiene < trans-1,3-pentadiene
3
Consider the hydrogenation reaction of each compound listed and rank the compounds in order of increasing ΔH° of this reaction. The most negative ΔH° should be listed first.
2,5-dimethyl-1,3-cycloheptadiene,
1,4-dimethyl-1,3-cycloheptadiene, and
3,6-dimethyl-1,4-cycloheptadiene
2,5-dimethyl-1,3-cycloheptadiene,
1,4-dimethyl-1,3-cycloheptadiene, and
3,6-dimethyl-1,4-cycloheptadiene
3,6-dimethyl-1,4-cycloheptadiene
< 2,5-dimethyl-1,3-cycloheptadiene
< 1,4-dimethyl-1,3-cycloheptadiene
< 2,5-dimethyl-1,3-cycloheptadiene
< 1,4-dimethyl-1,3-cycloheptadiene
4
What descriptive term is applied to the type of diene represented by 1,5-octadiene?
A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above
A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
Show how the carbon p orbitals overlap to form the lowest energy π molecular orbital of 1,3-butadiene.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
Draw (E)-1,3-hexadiene in its s-cis conformation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
When the relative energies of the s-cis and s-trans conformers of 1,3-butadiene are compared, one finds that ________.
A) the s-cis conformer is lower in energy than the s-trans
B) the s-trans conformer is lower in energy than the s-cis
C) the two conformers are of equal energy
A) the s-cis conformer is lower in energy than the s-trans
B) the s-trans conformer is lower in energy than the s-cis
C) the two conformers are of equal energy

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
In 1,3,5-hexatriene, there are ________ nodes in the HOMO and ________ nodes in the LUMO for this molecule.
A) 1; 2
B) 2; 3
C) 2; 0
D) 2; 1
A) 1; 2
B) 2; 3
C) 2; 0
D) 2; 1

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
What is the hybridization of the central carbon of allene (1,2-propadiene)?
A) sp
B) sp2
C) sp3
D) p
E) none of the above
A) sp
B) sp2
C) sp3
D) p
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
Which compound has the smallest heat of hydrogenation?
A) 5-methyl-1,2-hexadiene
B) (E)-5-methyl-1,3-hexadiene
C) 5-methyl-1,4-hexadiene
D) 2-methyl-1,5-hexadiene
E) (E)-2-methyl-2,4-hexadiene
A) 5-methyl-1,2-hexadiene
B) (E)-5-methyl-1,3-hexadiene
C) 5-methyl-1,4-hexadiene
D) 2-methyl-1,5-hexadiene
E) (E)-2-methyl-2,4-hexadiene

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
How many nodes, other than the node coincident with the molecular plane, are found in the highest energy π MO of 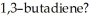
A) 0
B) 1
C) 2
D) 3
E) none of the above
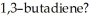
A) 0
B) 1
C) 2
D) 3
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
What is the resonance energy of a system?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds is the most stable?
A) (E)-2-methyl-1,3-pentadiene
B) 2-methyl-1,2-pentadiene
C) (Z)-2-methyl-1,3-pentadiene
D) 2-methyl-2,3-pentadiene
E) 2-methyl-1,4-pentadiene
A) (E)-2-methyl-1,3-pentadiene
B) 2-methyl-1,2-pentadiene
C) (Z)-2-methyl-1,3-pentadiene
D) 2-methyl-2,3-pentadiene
E) 2-methyl-1,4-pentadiene

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
Draw (Z)-1,3-hexadiene in its s-trans conformation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
Rank the following compounds in order of increasing heats of hydrogenation. (The smallest ΔH is first.) 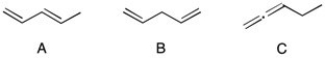
A) A < B < C
B) C < B < A
C) C < A < B
D) A < C < B

A) A < B < C
B) C < B < A
C) C < A < B
D) A < C < B

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
The number of π molecular orbitals in a molecule is always equal to the number of ________.
A) π bonds
B) p orbitals used to construct the π bonds
C) electrons in the π system
D) hydrogen atoms in the molecule
A) π bonds
B) p orbitals used to construct the π bonds
C) electrons in the π system
D) hydrogen atoms in the molecule

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
What descriptive term is applied to the type of diene represented by 2,4-hexadiene?
A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above
A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the hydrogenation of each compound listed and rank the compounds in order of increasing ΔH°. The most negative ΔH° should be listed first.
cis-pent-2-ene, 2,3-pentadiene, and trans-1,3-pentadiene
cis-pent-2-ene, 2,3-pentadiene, and trans-1,3-pentadiene

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
Among a series of isomeric trienes, the more negative the ΔH° of the hydrogenation reaction of a given triene, the ________ stable it is relative to the others in the isomeric series.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
What descriptive term is applied to the type of diene shown? 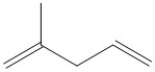
A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above

A) conjugated diene
B) cumulated diene
C) isolated diene
D) alkynyl diene
E) none of the above

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
Name the two major products which are formed when 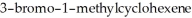
undergoes solvolysis in hot methanol.
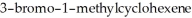
undergoes solvolysis in hot methanol.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
In the addition of HBr to conjugated dienes, is the product which results from 1,2-addition or that which results from 1,4-addition typically the product of kinetic control?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the structure of the major product which results from 1,2-addition of HBr to the diene shown below. 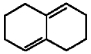


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
When 1 mole of anhydrous HCl is reacted with excess 1,3-pentadiene, both the 1,2 and the 1,4-addition products are formed. Which of the following structures shown below is the least likely to be one of these products? (Note: When a chiral carbon is formed in this reaction a racemic mixture results, only one of the two possible enantiomers is shown.)
A)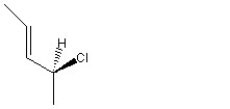
B)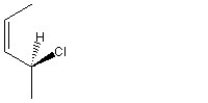
C)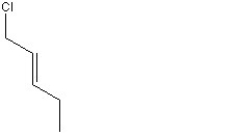
D)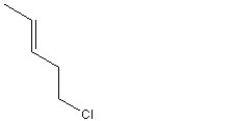
E)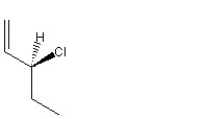
A)

B)
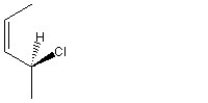
C)

D)

E)


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following alkyl halides yields the most stable carbocation intermediate during solvolysis in hot ethanol?
A) methyl iodide
B) (S)-2-bromopentane
C) (R)-2-bromopentane
D) (S)-3-bromopent-1-ene
E) 1-chlorobutane
A) methyl iodide
B) (S)-2-bromopentane
C) (R)-2-bromopentane
D) (S)-3-bromopent-1-ene
E) 1-chlorobutane

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
How many molecular orbitals are needed to represent the conjugated π system of β-carotene? 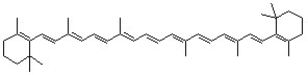
A) 1
B) 10
C) 11
D) 20
E) 22

A) 1
B) 10
C) 11
D) 20
E) 22

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
When 3-bromo-1-methylcyclohexene is heated in a good ionizing solvent, reactions occur through a carbocation intermediate. Draw all reasonable resonance contributors of this cation and indicate which is the major contributor.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
When 3-bromobut-1-ene is heated in a good ionizing solvent, reactions occur through a carbocation intermediate. Draw all reasonable resonance contributors of this cation and indicate which is the major contributor.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
Draw structures for the two major products of the following reaction. 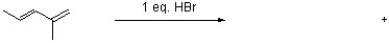


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
When (S)-3-bromopent-1-ene is heated in water, which of the following compounds is not produced?
A) (S)-pent-1-en-3-ol
B) (R)-pent-1-en-3-ol
C) pent-4-en-1-ol
D) (E)-pent-2-en-1-ol
E) (Z)-pent-2-en-1-ol
A) (S)-pent-1-en-3-ol
B) (R)-pent-1-en-3-ol
C) pent-4-en-1-ol
D) (E)-pent-2-en-1-ol
E) (Z)-pent-2-en-1-ol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the two major organic products of the following reaction. 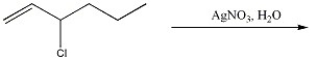


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the two major organic products of the following reaction. 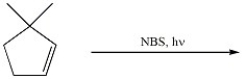
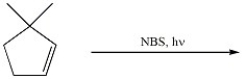

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following represents the highest occupied molecular orbital for the conjugated pi system in Vitamin D3? 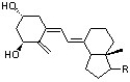
A)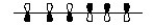
B)
C)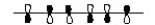
D)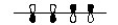

A)
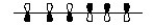
B)

C)
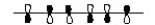
D)


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the LUMO of 1,3-butadiene.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
The structure of cholesterol is shown below. How many allylic carbons are there in this structure? 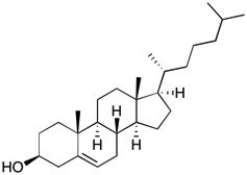
A) 2
B) 3
C) 4
D) 5

A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
Give a representation of the highest occupied π MO of 1,3-butadiene in its ground state.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
Provide a detailed, stepwise mechanism for the reaction shown below. 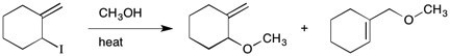


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the structure of the major product which results from 1,4-addition of Br2 to the diene shown below. 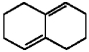


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
Give a representation of the lowest occupied π MO of 1,3-butadiene in its ground state.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the HOMO of 1,3-butadiene.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the HOMO of allyl anion.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the LUMO of pentadienyl anion.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
Give a representation of the bonding π molecular orbital of the allyl anion.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
Draw the structure of the major product which results when the diene shown is treated with HBr at -80°C. 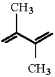


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
Show how the carbon p orbitals overlap to form the π2 molecular orbital of the allyl cation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the resonance structures of the intermediate and then predict the two major products in the following reaction. 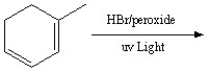


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the HOMO of pentadienyl anion.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
Provide the necessary synthetic sequence for preparing 1,3-cyclopentadiene from cyclopentane.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the 2 organic products expected from this reaction, label them as thermodynamic or kinetic and tell which predominates at -80°C. 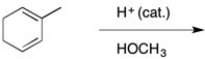


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
In the diene shown below, there are two possible double bonds that could react when 1 eq. of HBr is added. However, the double bond labeled "A" reacts preferentially to give the major product. Using resonance structures, justify this observation. 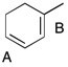


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
Including all possible stereoisomeric forms, how many distinct allylic bromides could be produced when 2-methylpent-1-ene is treated with NBS under irradiation by a sunlamp?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
In the allyl radical, which π molecular orbital is singly occupied?
A) The bonding π molecular orbital.
B) The nonbonding π molecular orbital.
C) The antibonding π molecular orbital.
D) None of the above.
A) The bonding π molecular orbital.
B) The nonbonding π molecular orbital.
C) The antibonding π molecular orbital.
D) None of the above.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
What is the major organic product which results when cycloheptene is irradiated in the presence of N-bromosuccinimide?
A) 1-bromocycloheptene
B) 2-bromocycloheptene
C) 1,2-dibromocycloheptane
D) 3-bromocycloheptene
E) 4-bromocycloheptene
A) 1-bromocycloheptene
B) 2-bromocycloheptene
C) 1,2-dibromocycloheptane
D) 3-bromocycloheptene
E) 4-bromocycloheptene

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the HOMO of pentadienyl cation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
Three halogenated organic products result from the reaction below. Draw the structure of each product and then circle the most stable product. 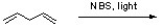
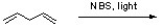

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the structure of the major product which results when the diene shown is treated with HBr at 40°C. 


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
What name is given to the type of "control" which arises in a reaction which does not achieve equilibrium and in which the product distribution is determined by the relative activation energies of the pathways which produced them?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
Give a representation of the antibonding π molecular orbital of the allyl cation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the LUMO of pentadienyl cation.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
Show how the carbon p orbitals overlap to form the LUMO of the allyl anion.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
What reagent could be used to convert allyl tosylate directly into oct-1-ene?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
Using resonance structures, explain the regiochemistry observed in this reaction: 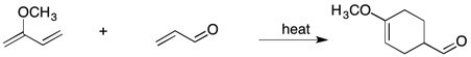
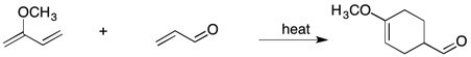

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following terms best describes a Diels-Alder reaction?
A) a [4+2] cycloaddition
B) a [2+2] cycloaddition
C) a sigmatropic rearrangement
D) a 1,3-dipolar cycloaddition
E) a substitution reaction
A) a [4+2] cycloaddition
B) a [2+2] cycloaddition
C) a sigmatropic rearrangement
D) a 1,3-dipolar cycloaddition
E) a substitution reaction

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
What diene and dienophile would react to give the product below? 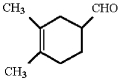


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
For what does the acronym HOMO stand?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
Draw mechanism for the following reaction: 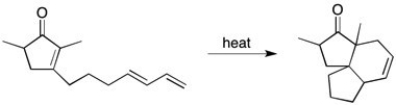


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
In a Diels-Alder reaction, what is the endo rule?

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
Explain why SN2 reactions on allyl bromide proceed faster than corresponding reactions on ethyl bromide.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the major organic product of the following reaction. 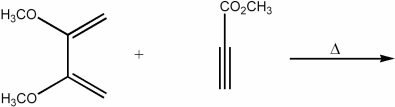


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
How many electrons are present in the nonbonding π molecular orbital of the allyl cation?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
Provide the structure of the major organic product in the following reaction. 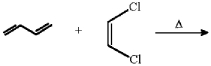


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
How many electrons are present in the nonbonding π molecular orbital of the allyl anion?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
The Diels-Alder reaction is a concerted reaction; this means ________.
A) a mixture of endo and exo products are formed
B) all bond making and bond breaking occurs simultaneously
C) the products contain rings
D) the reaction follows Markovnikov's rule
E) the reaction is highly endothermic
A) a mixture of endo and exo products are formed
B) all bond making and bond breaking occurs simultaneously
C) the products contain rings
D) the reaction follows Markovnikov's rule
E) the reaction is highly endothermic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following: 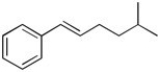


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
When 1,3-butadiene reacts with CH2

CHCN, which of the terms below best describes the product mixture?
A) a mixture of two diastereomers
B) a single compound
C) a racemic mixture
D) optically active
E) a mixture of bicyclic compounds

CHCN, which of the terms below best describes the product mixture?
A) a mixture of two diastereomers
B) a single compound
C) a racemic mixture
D) optically active
E) a mixture of bicyclic compounds

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
What diene and dienophile would react to give the product below? 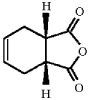


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
Provide the major organic product of the following reaction. 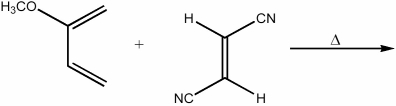


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the major organic product that results from the intramolecular Diels-Alder reaction. 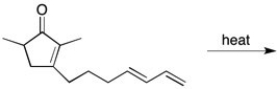


Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following compounds is the most reactive dienophile in a Diels-Alder reaction with 1,3-butadiene?
A) CH2

CHOCH3
B) CH2

CHCHO
C) CH3CH CHCH3
CHCH3
D) (CH3)2C CH2
CH2
E) CH2

CH2
A) CH2

CHOCH3
B) CH2

CHCHO
C) CH3CH
 CHCH3
CHCH3D) (CH3)2C
 CH2
CH2E) CH2

CH2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
Which substrate would react most rapidly in an SN2 reaction?
A) CH3CH2CH=CHCH2Br
B) BrCH2CH2CH=CHCH3
C) CH3CHBrCH=CH2CH3
D) CH3CH2CH2CH=CHBr
A) CH3CH2CH=CHCH2Br
B) BrCH2CH2CH=CHCH3
C) CH3CHBrCH=CH2CH3
D) CH3CH2CH2CH=CHBr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck


