Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
What reagent could be used to convert allyl tosylate directly into oct-1-ene?
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
n-pentyllithium or n-pentylmagnesium bromide
Consider the possible thermal [4+4] cycloaddition of two molecules of 1,3-butadiene to generate cycloocta-1,5-diene. Show the HOMO/LUMO interaction which would result, and use this interaction to predict whether the proposed cycloaddition could occur.
Free
(Essay)
4.9/5  (33)
(33)
Correct Answer:
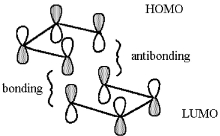 The cycloaddition is thermally forbidden.
The cycloaddition is thermally forbidden.
Provide the structure of the major organic product in the following reaction. 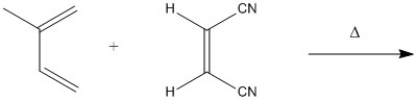
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
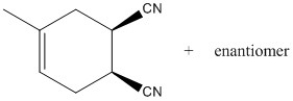
Three halogenated organic products result from the reaction below. Draw the structure of each product and then circle the most stable product. 
(Essay)
4.8/5  (34)
(34)
When 1,3-butadiene reacts with CH2
 CHCN, which of the terms below best describes the product mixture?
CHCN, which of the terms below best describes the product mixture?
(Multiple Choice)
4.8/5  (38)
(38)
Show how the carbon p orbitals overlap to form the π2 molecular orbital of the allyl cation.
(Essay)
4.8/5  (34)
(34)
Which diene reacts more rapidly in Diels-Alder reactions, cyclopentadiene or 1,3-butadiene? Briefly explain your choice.
(Essay)
4.9/5  (33)
(33)
Which compound requires a higher energy photon to cause a π to π* (HOMO/LUMO) transition, 1,3-butadiene or 1,3,5-hexatriene?
(Short Answer)
4.8/5  (33)
(33)
Assuming kinetic conditions, provide the correct stereochemistry and regiochemistry of the major product of the following reaction. 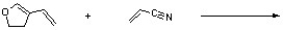
(Essay)
4.9/5  (35)
(35)
The structure of cholesterol is shown below. How many allylic carbons are there in this structure? 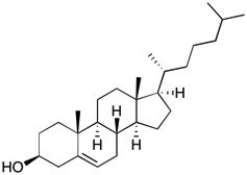
(Multiple Choice)
5.0/5  (23)
(23)
Provide the structure of the major organic product in the following reaction. 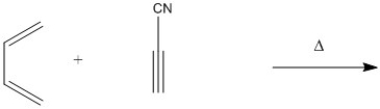
(Essay)
4.9/5  (31)
(31)
What descriptive term is applied to the type of diene shown? 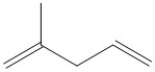
(Multiple Choice)
4.8/5  (34)
(34)
Consider the hydrogenation of each compound listed and rank the compounds in order of increasing ΔH°. The most negative ΔH° should be listed first.
cis-pent-2-ene, 2,3-pentadiene, and trans-1,3-pentadiene
(Essay)
4.9/5  (31)
(31)
Showing 1 - 20 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)