Deck 15: Carboxylic Acids and Nitriles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 15: Carboxylic Acids and Nitriles
1
Draw structures corresponding to each of the following IUPAC names.
Draw:
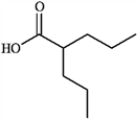
2-propylpentanoic acid
Draw:
2-propylpentanoic acid
2
Draw structures corresponding to each of the following IUPAC names.
Draw:
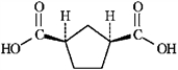
cis-cyclopentane-1,3-dicarboxylic acid
Draw:
cis-cyclopentane-1,3-dicarboxylic acid
3
Consider the data in the table below to answer the following question(s).
I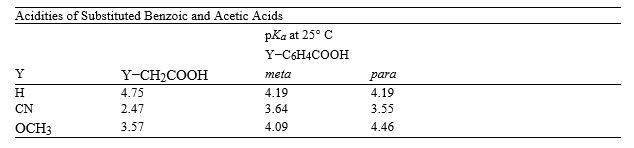
Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
I

Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Electron-withdrawing groups, like −CN and −OCH3, inductively withdraw electron density, which stabilizes the resulting carboxylate anion and thus increases the acidity of the carboxylic acid. These inductive effects are strongly dependent on distance. The −CN and −OCH3 substituents are closer to the carboxylate in the substituted acetic acids than in the substituted benzoic acids, so their effect is greater.
4
Consider the data in the table below to answer the following question(s).
I
Refer to instructions. Calculate the percent dissociation of 0.100 M solution of m-methoxybenzoic acid.
I

Refer to instructions. Calculate the percent dissociation of 0.100 M solution of m-methoxybenzoic acid.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):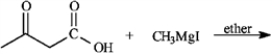
Give major product(s):
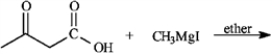

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the data in the table below to answer the following question(s).
I
Refer to instructions. Which of the acids in the table has the strongest conjugate base?
I

Refer to instructions. Which of the acids in the table has the strongest conjugate base?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
At pH 3.80 calculate the % in the dissociated and undissociated form in a 0.00200 M solution of pyruvic acid, pKa = 2.39.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
Draw structures corresponding to each of the following IUPAC names.
Draw:
cyanoacetic acid
Draw:
cyanoacetic acid

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
Draw structures corresponding to each of the following IUPAC names.
Draw:
2-chlorobenzoic acid
Draw:
2-chlorobenzoic acid

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to instructions. This reaction can be described as a _____ process.
A) carbonylation
B) carboxylation
C) carbaniolation
D) cationation
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to instructions. This reaction can be described as a _____ process.
A) carbonylation
B) carboxylation
C) carbaniolation
D) cationation

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):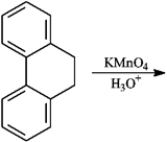
Give major product(s):


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
Draw structures corresponding to each of the following IUPAC names.
Draw:
prop-2-enenitrile
Draw:
prop-2-enenitrile

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
What is the order of increasing reactivity for the following compounds in electrophillic aromatic substitution reactions? (least to most reactive)
PKa
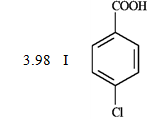
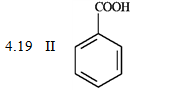
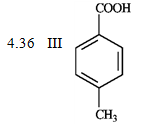
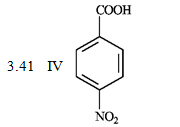
A) IV, I, II, III
B) III, II, I, IV
C) II, III, I, IV
D) III, II, i, IV
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
What is the order of increasing reactivity for the following compounds in electrophillic aromatic substitution reactions? (least to most reactive)
PKa




A) IV, I, II, III
B) III, II, I, IV
C) II, III, I, IV
D) III, II, i, IV

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):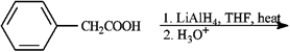
Give major product(s):
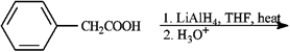

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to instructions. Write the complete reaction sequence for the process described above.
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
Refer to instructions. Write the complete reaction sequence for the process described above.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
What is the order of increasing acidity for the following compounds? (least to most)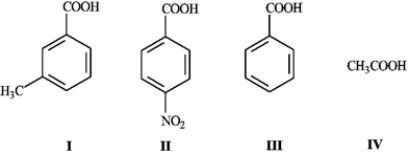
A) IV, I, III, II
B) IV, II, III, I
C) II, III, I, IV
D) I, III, II, IV
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
What is the order of increasing acidity for the following compounds? (least to most)

A) IV, I, III, II
B) IV, II, III, I
C) II, III, I, IV
D) I, III, II, IV

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
An ether solution contains the following components.
2,3,4,5-tetramethylbenzene benzyl alcohol 2-hydroxybenzoic acid
If this solution is extracted with a 5% solution of NaHCO3, that is immiscible with ether, which components end up in the ether layer and which in the NaHCO3 layer?
2,3,4,5-tetramethylbenzene benzyl alcohol 2-hydroxybenzoic acid
If this solution is extracted with a 5% solution of NaHCO3, that is immiscible with ether, which components end up in the ether layer and which in the NaHCO3 layer?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the data in the table below to answer the following question(s).
I
Refer to instructions. Draw the structure of the strongest acid in the table.
I

Refer to instructions. Draw the structure of the strongest acid in the table.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
Draw structures corresponding to each of the following IUPAC names.
Draw:
phenylacetonitrile or phenylethanenitrile
Draw:
phenylacetonitrile or phenylethanenitrile

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Give major product(s):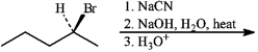
Give major product(s):
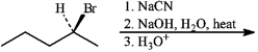

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major organic product obtained from the following reaction? 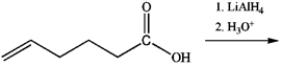
A)
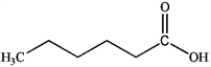
B)

C)

D)
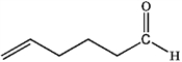

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would represent the correct reaction conditions for the following conversion? 
A) 1) KMnO4, 2) LiAlH4
B) 1) Mg, ether, 2) CO2, 3) LiAlH4
C) 1) NaOH, H2O, 2) LiAlH4
D) 1) SOCl2, benzene, 2) LiAlH4
E) either b or c

A) 1) KMnO4, 2) LiAlH4
B) 1) Mg, ether, 2) CO2, 3) LiAlH4
C) 1) NaOH, H2O, 2) LiAlH4
D) 1) SOCl2, benzene, 2) LiAlH4
E) either b or c

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
Complete the following reaction sequence with the missing major organic products.
Complete the following reaction sequence with the missing major organic products.


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major organic product obtained from the following reaction? 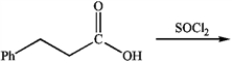
A)
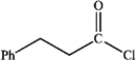
B)
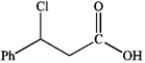
C)
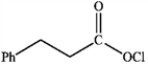
D)
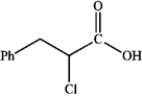

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
The product shown can be synthesized by two different pathways as shown by the reaction conditions. Draw the structure of starting materials that should be placed in each of the boxes. 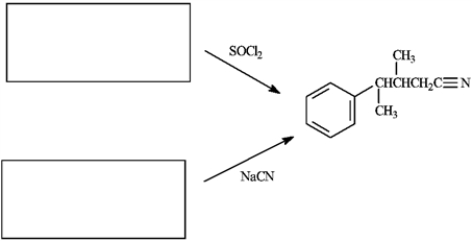


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
What is the major organic product obtained from the following reaction? 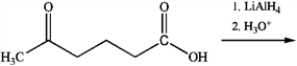
A)
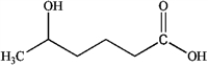
B)
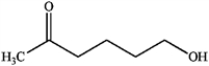
C)
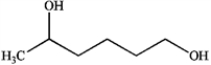
D)
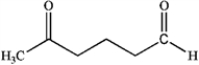

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the correct order of decreasing acid strength (more acidic > less acidic)?
A) FCH2COOH > CH3COOH > F2CHCOOH
B) FCH2COOH > CH3COOH > CH3OH
C) CH3CH2OH > ClCH2COOH > BrCH2COOH
D) CH3COOH > ClCH2COOH > CH3OH
A) FCH2COOH > CH3COOH > F2CHCOOH
B) FCH2COOH > CH3COOH > CH3OH
C) CH3CH2OH > ClCH2COOH > BrCH2COOH
D) CH3COOH > ClCH2COOH > CH3OH

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name of the following compound? 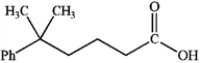
A) 5,5-dimethyl-5-phenylpentanoic acid
B) 5-methyl-5-phenylhexanoic acid
C) 2,2-dimethylphenylpropanoic acid
D) 5,5-dimethyl-5-phenylbutanal

A) 5,5-dimethyl-5-phenylpentanoic acid
B) 5-methyl-5-phenylhexanoic acid
C) 2,2-dimethylphenylpropanoic acid
D) 5,5-dimethyl-5-phenylbutanal

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following has the highest boiling point?
A) butanoic acid
B) butan-2-ol
C) hexanoic acid
D) heptan-3-one
A) butanoic acid
B) butan-2-ol
C) hexanoic acid
D) heptan-3-one

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following 1H NMR to answer the following question. 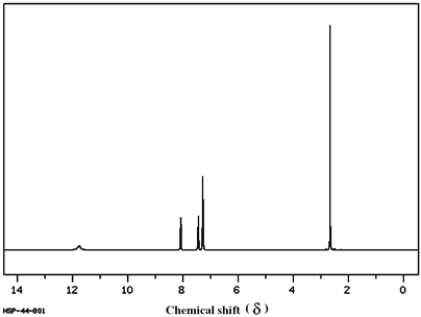
Refer to instructions. This compound is known to contain both a −C=O group and an −OH group. Based on this spectrum, this compound would be classified as:
A) aromatic carboxylic acid
B) aliphatic carboxylic acid
C) carbonyl containing an alcohol functional group
D) either a or b

Refer to instructions. This compound is known to contain both a −C=O group and an −OH group. Based on this spectrum, this compound would be classified as:
A) aromatic carboxylic acid
B) aliphatic carboxylic acid
C) carbonyl containing an alcohol functional group
D) either a or b

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
Choose best method: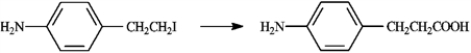
Choose best method:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
Refer to instructions and answer the following questions.
a)Give major product(s): b)Draw the structure of the intermediate product(s) that form(s).
b)Draw the structure of the intermediate product(s) that form(s).
Refer to instructions and answer the following questions.
a)Give major product(s):
 b)Draw the structure of the intermediate product(s) that form(s).
b)Draw the structure of the intermediate product(s) that form(s).
Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
Choose best method: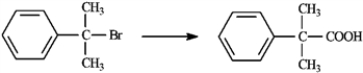
Choose best method:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
Draw the structure of (Z)-4-methylhex-3-enoic acid.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following does not represent a similarity between nitriles and carboxylic acids?
A) contain 3 carbon bonds to an electronegative element
B) contain two π bonds.
C) act as electrophiles
D) undergo nucleophilic substitution reactions
E) all of these are characteristic of both nitriles and carboxylic acids.
A) contain 3 carbon bonds to an electronegative element
B) contain two π bonds.
C) act as electrophiles
D) undergo nucleophilic substitution reactions
E) all of these are characteristic of both nitriles and carboxylic acids.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
Based on the following IR spectrum, this compound would be classified as: 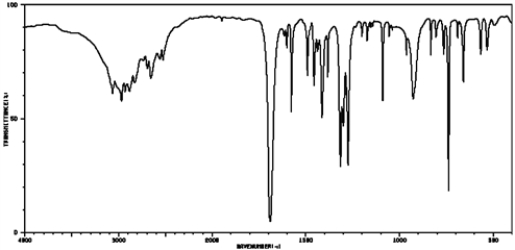
A) aliphatic carboxylic acid
B) aromatic carboxylic acid
C) aliphatic nitrile
D) aromatic nitrile
E) carbonyl containing an alcohol functional group

A) aliphatic carboxylic acid
B) aromatic carboxylic acid
C) aliphatic nitrile
D) aromatic nitrile
E) carbonyl containing an alcohol functional group

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck


