Exam 15: Carboxylic Acids and Nitriles
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds; Acids and Bases41 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry29 Questions
Exam 5: Stereochemistry at Tetrahedral Centers40 Questions
Exam 6: An Overview of Organic Reactions39 Questions
Exam 7: Alkenes and Alkynes36 Questions
Exam 8: Reactions of Alkenes and Alkynes38 Questions
Exam 9: Aromatic Compounds37 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy42 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy43 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy41 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations43 Questions
Exam 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides38 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions36 Questions
Exam 15: Carboxylic Acids and Nitriles36 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions46 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions46 Questions
Exam 18: Amines and Heterocycles36 Questions
Exam 19: Biomolecules: Amino Acids, Peptides, and Proteins52 Questions
Exam 20: Amino Acid Metabolism32 Questions
Exam 21: Biomolecules: Carbohydrates49 Questions
Exam 22: Carbohydrate Metabolism45 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism42 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism34 Questions
Exam 26: Orbitals and Organic Chemistry: Pericyclic Reactionse44 Questions
Exam 27: Synthetic Polymerse35 Questions
Select questions type
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
-Choose best method: 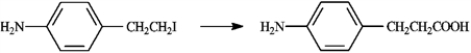
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
Amine protons interfere with the formation of Grignard reagents, so nitrile hydrolysis is the best method for carrying out this conversion.
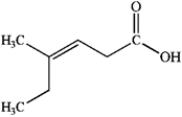
Draw the structure of (Z)-4-methylhex-3-enoic acid.
Free
(Essay)
4.9/5  (42)
(42)
Correct Answer:
Consider the data in the table below to answer the following question(s).
I 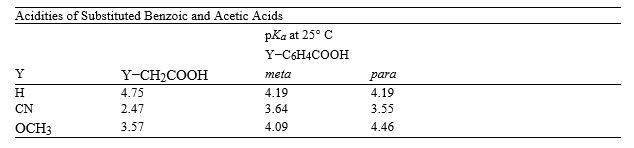 -Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
-Refer to instructions. Explain why cyanoacetic acid and methoxyacetic acid are more acidic than their correspondingly substituted benzoic acid counterparts.
Free
(Essay)
4.9/5  (43)
(43)
Correct Answer:
Electron-withdrawing groups, like −CN and −OCH3, inductively withdraw electron density, which stabilizes the resulting carboxylate anion and thus increases the acidity of the carboxylic acid. These inductive effects are strongly dependent on distance. The −CN and −OCH3 substituents are closer to the carboxylate in the substituted acetic acids than in the substituted benzoic acids, so their effect is greater.
Which of the following does not represent a similarity between nitriles and carboxylic acids?
(Multiple Choice)
4.9/5  (27)
(27)
The product shown can be synthesized by two different pathways as shown by the reaction conditions. Draw the structure of starting materials that should be placed in each of the boxes. 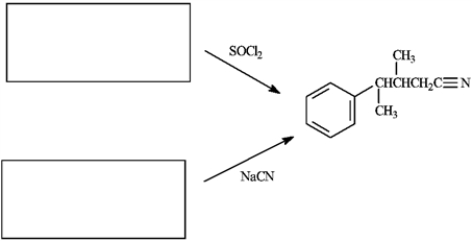
(Essay)
4.7/5  (39)
(39)
An ether solution contains the following components.
2,3,4,5-tetramethylbenzene benzyl alcohol 2-hydroxybenzoic acid
If this solution is extracted with a 5% solution of NaHCO3, that is immiscible with ether, which components end up in the ether layer and which in the NaHCO3 layer?
(Essay)
4.7/5  (28)
(28)
What is the major organic product obtained from the following reaction? 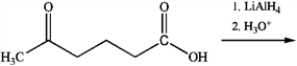
(Multiple Choice)
4.9/5  (38)
(38)
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
-Give major product(s): 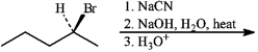
(Essay)
4.9/5  (40)
(40)
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
-Choose best method: 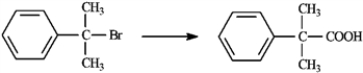
(Essay)
4.8/5  (32)
(32)
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
-What is the order of increasing reactivity for the following compounds in electrophillic aromatic substitution reactions? (least to most reactive)
PKa
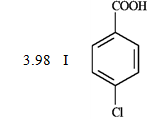
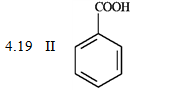
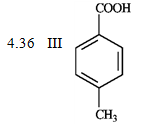
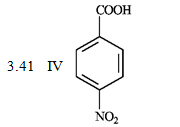
(Multiple Choice)
4.8/5  (39)
(39)
Consider the data below to answer the following question(s).
When CO2 is bubbled through an ether solution of benzylmagnesium bromide and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step.
-Refer to instructions. This reaction can be described as a _____ process.
(Multiple Choice)
4.9/5  (38)
(38)
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
-Refer to instructions and answer the following questions.
a)Give major product(s):  b)Draw the structure of the intermediate product(s) that form(s).
b)Draw the structure of the intermediate product(s) that form(s).
(Essay)
4.8/5  (36)
(36)
Draw structures corresponding to each of the following IUPAC names.
-Draw:
phenylacetonitrile or phenylethanenitrile
(Essay)
4.9/5  (48)
(48)
Based on the following IR spectrum, this compound would be classified as: 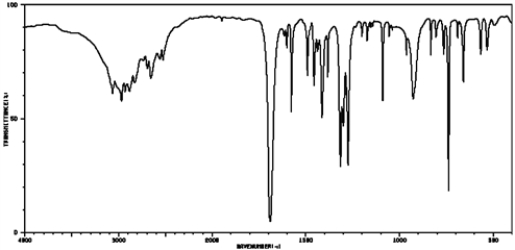
(Multiple Choice)
4.9/5  (33)
(33)
At pH 3.80 calculate the % in the dissociated and undissociated form in a 0.00200 M solution of pyruvic acid, pKa = 2.39.
(Short Answer)
4.9/5  (35)
(35)
Consider the data in the table below to answer the following question(s).
I  -Refer to instructions. Which of the acids in the table has the strongest conjugate base?
-Refer to instructions. Which of the acids in the table has the strongest conjugate base?
(Short Answer)
4.8/5  (33)
(33)
Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry.
-Give major product(s): 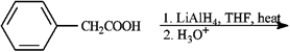
(Essay)
4.9/5  (32)
(32)
Carboxylic acids are synthesized from alkyl halides via Grignard reagent carboxylation or nitrile hydrolysis. Choose the best method for affecting each of the following conversions. Explain each of your choices. If neither method is appropriate, explain.
-Complete the following reaction sequence with the missing major organic products. 
(Essay)
4.8/5  (36)
(36)
Draw structures corresponding to each of the following IUPAC names.
-Draw:
2-propylpentanoic acid
(Essay)
4.9/5  (36)
(36)
Consider the data in the table below to answer the following question(s).
I  -Refer to instructions. Calculate the percent dissociation of 0.100 M solution of m-methoxybenzoic acid.
-Refer to instructions. Calculate the percent dissociation of 0.100 M solution of m-methoxybenzoic acid.
(Short Answer)
4.8/5  (24)
(24)
Showing 1 - 20 of 36
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)