Deck 14: Aldehydes and Ketones: Nucleophilic Addition Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 14: Aldehydes and Ketones: Nucleophilic Addition Reactions
1
Draw structures corresponding to each of the following names.
Draw:
cyclohexen-2-one
Draw:
cyclohexen-2-one
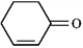
2
Consider the reaction below to answer the following question. 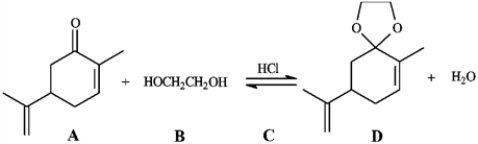
Refer to instructions. The product of this reaction is called:
A) an ylide
B) an acetal
C) a gem diol
D) a hydrate

Refer to instructions. The product of this reaction is called:
A) an ylide
B) an acetal
C) a gem diol
D) a hydrate
an acetal
3
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Interpret the mass spectral data.
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Interpret the mass spectral data.
A fragment at m/z = 71 indicates a loss of 43, or a C3H7 group, from α-cleavage. A fragment at m/z = 86 indicates a loss of 28, or an ethylene group, from McLafferty rearrangement (transfer of a hydrogen atom from the gamma carbon to the carbonyl oxygen with concommitant breaking of the bond between the alpha and beta carbon).
4
Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.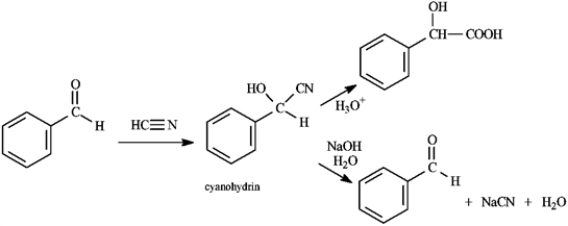
Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
A) a nucleophilic substitution
B) an electrophilic addition
C) an electrophilic substitution
D) a nucleophilic addition
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.

Refer to instructions. The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
A) a nucleophilic substitution
B) an electrophilic addition
C) an electrophilic substitution
D) a nucleophilic addition

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
Predict the products from the information given for the following question(s).
Predict: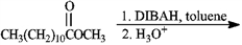
Predict:
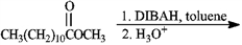

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
α,β-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the β carbon, as shown below. Use this information to answer the following question(s). 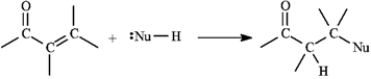
Refer to instructions. This reaction is called a(n) _____ reaction.
A) conjugate addition.
B) electrophilic addition.
C) direct addition
D) 1,2-addition.
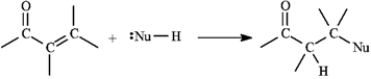
Refer to instructions. This reaction is called a(n) _____ reaction.
A) conjugate addition.
B) electrophilic addition.
C) direct addition
D) 1,2-addition.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
α,β-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the β carbon, as shown below. Use this information to answer the following question(s). 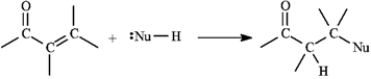
Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
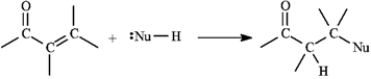
Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the products from the information given for the following question(s).
Predict: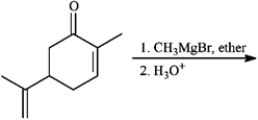
Predict:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Interpret the 1H NMR data.
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Interpret the 1H NMR data.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
Draw structures corresponding to each of the following names.
Draw:
benzophenone
Draw:
benzophenone

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Calculate the degree of unsaturation for this compound.
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Calculate the degree of unsaturation for this compound.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the reaction below to answer the following question. 
Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.

Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. What functional group is indicated by the IR data?
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. What functional group is indicated by the IR data?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.
Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Cyanohydrins are important intermediates in the synthesis of α-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. When a cyanohydrin is treated with aqueous base, however, the original carbonyl compound is isolated.

Refer to instructions. Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the reaction below to answer the following question. 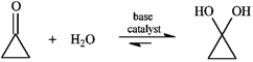
Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by:
A) making the carbonyl group more electrophilic
B) shifting the equilibrium of the reaction
C) making the carbonyl group less electrophilic
D) converting the water to hydroxide ion, a much better nucleophile

Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by:
A) making the carbonyl group more electrophilic
B) shifting the equilibrium of the reaction
C) making the carbonyl group less electrophilic
D) converting the water to hydroxide ion, a much better nucleophile

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the reaction below to answer the following question. 
Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.

Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
Draw structures corresponding to each of the following names.
Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)
Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
Provide IUPAC names for each structure below.
Name: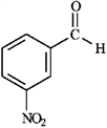
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the reaction below to answer the following question. 
The substance formed in the following reaction is called: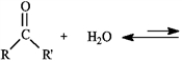
A) vicinal diol
B) geminal diol
C) acetal
D) hydrate
E) b or d

The substance formed in the following reaction is called:
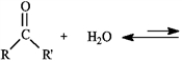
A) vicinal diol
B) geminal diol
C) acetal
D) hydrate
E) b or d

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
Provide IUPAC names for each structure below.
Name: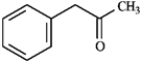
Name:


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of the following compound? 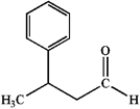
A) 3-methyl-3-phenylpropanol
B) 3-phenylbutanal
C) 3-phenyl-1-butanone
D) 3-phenylbutanoic acid

A) 3-methyl-3-phenylpropanol
B) 3-phenylbutanal
C) 3-phenyl-1-butanone
D) 3-phenylbutanoic acid

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
What is the name of the major organic product obtained from the following reaction? 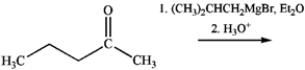
A) 2,3-dimethylheptan-3-ol
B) 2,4-dimethylheptan-4-ol
C) 3,5-dimethylheptan-4-ol
D) 3,5-dimethylheptan-3-ol

A) 2,3-dimethylheptan-3-ol
B) 2,4-dimethylheptan-4-ol
C) 3,5-dimethylheptan-4-ol
D) 3,5-dimethylheptan-3-ol

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
The nucleophillic addition of water to an aldehyde or ketone
A) is irreversible.
B) dependent on the structure of the carbonyl.
C) favored by neutral conditions.
D) produces a stable tetrahedral product.
E) all of these.
A) is irreversible.
B) dependent on the structure of the carbonyl.
C) favored by neutral conditions.
D) produces a stable tetrahedral product.
E) all of these.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
What is the IUPAC name of the following compound? 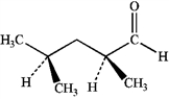
A) (2S,4R)-dimethylpentanal
B) (2S,4S)-dimethylpentanal
C) (R)-2,4-dimethylpentanal
D) (S)-2,4-dimethylpentanal

A) (2S,4R)-dimethylpentanal
B) (2S,4S)-dimethylpentanal
C) (R)-2,4-dimethylpentanal
D) (S)-2,4-dimethylpentanal

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
In the box, what is the name of the reactant used in the following reaction? 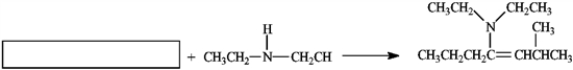
A) 2-methylhept-4-ene
B) heptan-4-one
C) 2-methylheptan-4-one
D) 2-methylhep-3-ene-4-one

A) 2-methylhept-4-ene
B) heptan-4-one
C) 2-methylheptan-4-one
D) 2-methylhep-3-ene-4-one

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the product(s) of the following reaction. 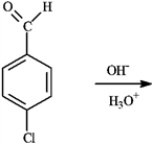


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the products of the following reactions. 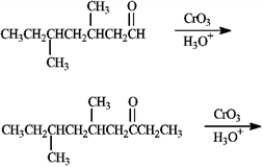


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct assignment of the functional groups in each of the following compounds? 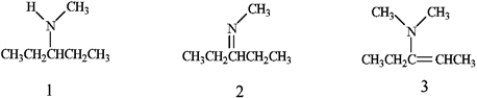
A) 1 = amine; 2 = imine; 3 = enamine
B) 1 = enamine; 2 = imine; 3 = amine
C) 1 = imine; 2 = enamine; 3 = amine
D) 1 = amine; 2 =enamine; 3 = imine

A) 1 = amine; 2 = imine; 3 = enamine
B) 1 = enamine; 2 = imine; 3 = amine
C) 1 = imine; 2 = enamine; 3 = amine
D) 1 = amine; 2 =enamine; 3 = imine

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
Draw the structure of the product obtained from the following reaction. 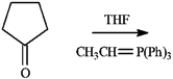
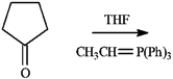

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following would correctly describe the respective 13C NMR and 1H NMR spectra for the indicated atoms for the compound shown below? 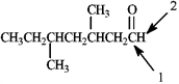
A) Atom 1 would produce a peak at 205 and atom 2 would appear as a singlet.
B) Atom 1 would produce a peak at 195 and atom 2 would appear as a singlet.
C) Atom 1 would produce a peak at 205 and atom 2 would appear as a triplet.
D) Atom 1 would produce a peak at 195 and atom 2 would appear as a triplet.

A) Atom 1 would produce a peak at 205 and atom 2 would appear as a singlet.
B) Atom 1 would produce a peak at 195 and atom 2 would appear as a singlet.
C) Atom 1 would produce a peak at 205 and atom 2 would appear as a triplet.
D) Atom 1 would produce a peak at 195 and atom 2 would appear as a triplet.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Propose a structure consistent with the spectral data presented above.
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
Refer to instructions. Propose a structure consistent with the spectral data presented above.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
Predict whether the following reactions of the depicted carbonyl containing compounds and amines will result in the formation of an enamine or an imine.
A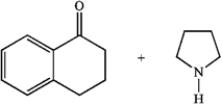 B
B 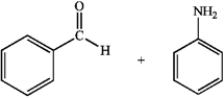 C
C 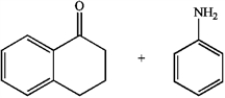
A
 B
B  C
C 

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
What hemiacetal would form from the internal nucleophillic addition reaction of the following compound? 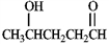
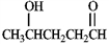

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
Predict the products of the following reactions. 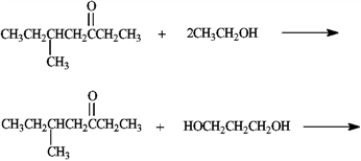


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
Synthesize the following alkene through the Wittig reaction of a carbonyl compound and a phosphorus ylide. 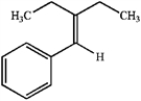


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
Based on the following IR spectrum, 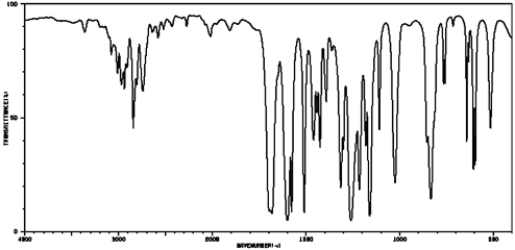
A) aromatic ketone
B) aromatic aldehyde
C) aliphatic ketone
D) aliphatic aldehyde

A) aromatic ketone
B) aromatic aldehyde
C) aliphatic ketone
D) aliphatic aldehyde

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck


