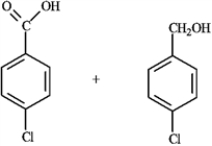
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds; Acids and Bases41 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry29 Questions
Exam 5: Stereochemistry at Tetrahedral Centers40 Questions
Exam 6: An Overview of Organic Reactions39 Questions
Exam 7: Alkenes and Alkynes36 Questions
Exam 8: Reactions of Alkenes and Alkynes38 Questions
Exam 9: Aromatic Compounds37 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy42 Questions
Exam 10: Structure Determination: Mass Spectrometry, Infrared Spectroscopy, and Ultraviolet Spectroscopy43 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy41 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations43 Questions
Exam 13: Alcohols, Phenols, and Thiols; Ethers and Sulfides38 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions36 Questions
Exam 15: Carboxylic Acids and Nitriles36 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions46 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions46 Questions
Exam 18: Amines and Heterocycles36 Questions
Exam 19: Biomolecules: Amino Acids, Peptides, and Proteins52 Questions
Exam 20: Amino Acid Metabolism32 Questions
Exam 21: Biomolecules: Carbohydrates49 Questions
Exam 22: Carbohydrate Metabolism45 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism42 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism34 Questions
Exam 26: Orbitals and Organic Chemistry: Pericyclic Reactionse44 Questions
Exam 27: Synthetic Polymerse35 Questions
Select questions type
Draw the structure of the product obtained from the following reaction. 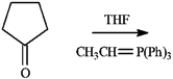
Free
(Essay)
4.8/5  (35)
(35)
Correct Answer:
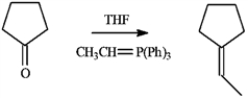
Consider the reaction below to answer the following question. 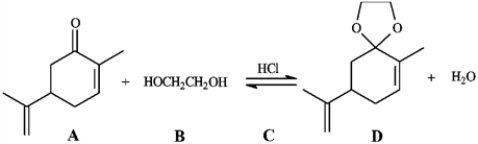 -Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
-Refer to instructions. The electrophile, the nucleophile and the catalyst in this reaction are indicated by letters _____, _____, and _____, respectively.
Free
(Short Answer)
4.8/5  (36)
(36)
Correct Answer:
A, B and C
Consider the reaction below to answer the following question. 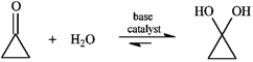 -Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
-Refer to instructions. Write the complete stepwise mechanism for the reaction shown above. Show all intermediate structures and all electron flow with arrows.
(Essay)
4.8/5  (39)
(39)
Consider the reaction below to answer the following question.  -The substance formed in the following reaction is called:
-The substance formed in the following reaction is called: 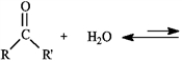
(Multiple Choice)
4.7/5  (37)
(37)
Consider the reaction below to answer the following question.  -Refer to instructions. The product of this reaction is called:
-Refer to instructions. The product of this reaction is called:
(Multiple Choice)
4.9/5  (39)
(39)
Predict the products from the information given for the following question(s).
-Predict: 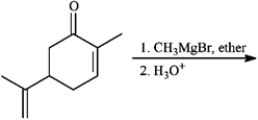
(Essay)
4.8/5  (41)
(41)
Consider the reaction below to answer the following question.  -Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by:
-Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by:
(Multiple Choice)
4.7/5  (35)
(35)
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
-Refer to instructions. Propose a structure consistent with the spectral data presented above.
(Essay)
4.8/5  (36)
(36)
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
-Refer to instructions. Calculate the degree of unsaturation for this compound.
(Essay)
4.7/5  (36)
(36)
α,β-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the β carbon, as shown below. Use this information to answer the following question(s). 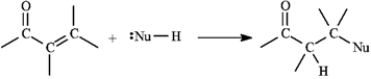 -Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
-Refer to instructions. Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
(Essay)
4.9/5  (47)
(47)
Consider the data below to answer the following question(s).
C7H14O
IR:
1715 cm−1
MS:
M+ at m/z = 114, α-cleavage fragment at m/z = 71.
McLafferty rearrangement fragment at m/z = 86.
1H NMR:
0.92 δ (6 H, triplet), 1.59 δ (4 H, multiplet), 2.36 δ (4 H, triplet)
-Refer to instructions. What functional group is indicated by the IR data?
(Essay)
4.9/5  (39)
(39)
What is the name of the major organic product obtained from the following reaction? 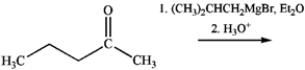
(Multiple Choice)
4.8/5  (36)
(36)
What hemiacetal would form from the internal nucleophillic addition reaction of the following compound? 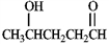
(Essay)
4.8/5  (30)
(30)
Draw structures corresponding to each of the following names.
-Draw:
5,5-dimethylcyclohexane-1,3-dione (dimedone)
(Essay)
4.8/5  (28)
(28)
Showing 1 - 20 of 36
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)