Deck 16: A Macroscopic Description of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/29
Play
Full screen (f)
Deck 16: A Macroscopic Description of Matter
1
3.00 moles of an ideal gas at a pressure of 250 kPa are held in a container of volume of 25.0 L. The ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K, and 1 atm = 1.01 x 105 Pa. The temperature of this gas is closest to
A) 240°C.
B) -180°C.
C) 480°C.
D) -1.0°C.
E) -22°C.
A) 240°C.
B) -180°C.
C) 480°C.
D) -1.0°C.
E) -22°C.
-22°C.
2
A weather balloon contains 12.0 m3 of hydrogen gas when the balloon is released from a location at which the temperature is 22.0°C and the pressure is 101 kPa. The balloon rises to a location where the temperature is -30.0°C and the pressure is 20.0 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon? Assume that in both cases the hydrogen gas is in thermal equilibrium with the outside air.
A) 14.0 m3
B) 2.38 m3
C) 49.9 m3
D) 82.6 m3
E) 4.16 m3
A) 14.0 m3
B) 2.38 m3
C) 49.9 m3
D) 82.6 m3
E) 4.16 m3
49.9 m3
3
For a fixed amount of gas, if the absolute temperature of the gas is doubled, what happens to the pressure of the gas?
A) The answer cannot be determined without volume information.
B) The pressure of the gas becomes double the original pressure.
C) The pressure of the gas becomes eight times the original pressure.
D) The pressure of the gas becomes one half the original pressure.
E) The pressure of the gas becomes four times the original pressure.
A) The answer cannot be determined without volume information.
B) The pressure of the gas becomes double the original pressure.
C) The pressure of the gas becomes eight times the original pressure.
D) The pressure of the gas becomes one half the original pressure.
E) The pressure of the gas becomes four times the original pressure.
The answer cannot be determined without volume information.
4
A bag of potato chips contains 2.00 L of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0°C. What will be the volume of the air in the bag if you take it with you, still sealed, to the mountains where the temperature is 7.00°C and atmospheric pressure is 70.0 kPa? Assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air. (1 atm = 1.01 × 105 Pa)
A) 4.13 L
B) 1.01 L
C) 1.38 L
D) 2.76 L
A) 4.13 L
B) 1.01 L
C) 1.38 L
D) 2.76 L

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
5
A sealed 26- m3 tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
6
Sometimes an experiment requires a certain pure gas to be used at reduced pressure. One way to achieve this is to purchase a sealed glass container filled with the gas, and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet. If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 2.0 L, what will the pressure be after the gas, which is to be assumed to be an ideal gas, is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
7
A hot air balloon has a volume of 2.00 × 103 m3 when fully inflated, and the air inside the balloon is always at atmospheric pressure of 1.01 × 105 Pa because of the large opening used to fill the balloon and heat the air inside it. What is the mass of hot air inside the balloon if its temperature is 120°C? The universal gas constant is 8.314 J/mol•K. (Assume a molecular weight of 28.8 g/mol for air.)
A) 1780 kg
B) 5850 kg
C) 203 kg
D) 62.0 kg
A) 1780 kg
B) 5850 kg
C) 203 kg
D) 62.0 kg

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
8
If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 1.2 x 104 Pa on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol · K and Avogadro's number is 6.022 × 1023 molecules/mol.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
9
A fixed amount of ideal gas is held in a rigid container that expands negligibly when heated. At 20°C the gas pressure is p. If we add enough heat to increase the temperature from 20°C to 40°C, the pressure will be
A) impossible to determine since we do not know the number of moles of gas in the container.
B) greater than 2p.
C) less than 2p.
D) equal to 2p.
E) impossible to determine since we do not know the volume of gas in the container.
A) impossible to determine since we do not know the number of moles of gas in the container.
B) greater than 2p.
C) less than 2p.
D) equal to 2p.
E) impossible to determine since we do not know the volume of gas in the container.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
10
Which contains more moles of material: 80 grams of helium gas (He, having atomic weight 4.0 g/mol) or 400 grams of argon gas (Ar, having atomic weight 40 g/mol)?
A) helium
B) argon
C) Both contain the same number of moles.
A) helium
B) argon
C) Both contain the same number of moles.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
11
A sealed 89-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
12
2.0 L of a ideal nitrogen gas (N2) are at 0.00°C and 1.0 atm. The ideal gas constant is R = 8.314 J/mol · K = 0.0821 L ∙ atm/mol ∙ K, Avogadro's number is 6.022 × 1023 molecules/mol, and the ATOMIC mass of nitrogen is 14 g/mol.
(a) Determine the number of moles of N2.
(b) How many molecules of N2 are present?
(c) What is the mass of this gas?
(a) Determine the number of moles of N2.
(b) How many molecules of N2 are present?
(c) What is the mass of this gas?

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
13
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket. The open end of the tube is under the water of a lake, as shown in the figure. When the lower end of the tube is just under the surface of the lake, where the temperature is 37°C and the pressure is 1.0 × 105 Pa, the air pocket occupies a volume of 630 cm3. Suppose now that the lower end of the tube is at a depth of 86 m in the lake, where the temperature is 7.0°C. What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 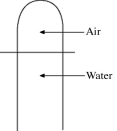


Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
14
An ideal gas is at a pressure 1.00 × 105 N/m2 and occupies a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant, what will be the new pressure in the gas?
A) 0.500 × 105 N/m2
B) 4.00 × 105 N/m2
C) 1.00 × 105 N/m2
D) 2.00 × 105 N/m2
E) The answer depends on the mass of the gas particles.
A) 0.500 × 105 N/m2
B) 4.00 × 105 N/m2
C) 1.00 × 105 N/m2
D) 2.00 × 105 N/m2
E) The answer depends on the mass of the gas particles.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
15
A cold trap is set up to cause molecules to linger near the suction in a vacuum system. If the cold trap has an effective volume of 0.200 L and is maintained at 13.0 K, how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol•K. Assume the behavior of an ideal gas.)
A) 1.11 × 1019 molecules
B) 1.10 × 1022 molecules
C) 7.71 × 1020 molecules
D) 7.71 × 1023 molecules
A) 1.11 × 1019 molecules
B) 1.10 × 1022 molecules
C) 7.71 × 1020 molecules
D) 7.71 × 1023 molecules

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
16
The number of molecules in one mole of a substance
A) depends on the molecular weight of the substance.
B) depends on the atomic weight of the substance.
C) depends on the density of the substance.
D) depends on the temperature of the substance.
E) is the same for all substances.
A) depends on the molecular weight of the substance.
B) depends on the atomic weight of the substance.
C) depends on the density of the substance.
D) depends on the temperature of the substance.
E) is the same for all substances.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
17
(a) Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Celsius and Kelvin scales?
(b) Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c) The boiling point of liquid nitrogen is 77.0 K. What is the corresponding temperature in the Fahrenheit and Celsius scales?
(b) Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c) The boiling point of liquid nitrogen is 77.0 K. What is the corresponding temperature in the Fahrenheit and Celsius scales?

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
18
A 3.2-L volume of neon gas (Ne) is at a pressure of 3.3 atm and a temperature of 330 K. The atomic mass of neon is 20.2 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The mass of the neon gas is closest to
A) 7.9 × 10-3 kg.
B) 4.6 × 10-3 kg.
C) 3.8 kg.
D) 7.8 kg.
E) 7.8 × 102 kg.
A) 7.9 × 10-3 kg.
B) 4.6 × 10-3 kg.
C) 3.8 kg.
D) 7.8 kg.
E) 7.8 × 102 kg.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
19
A sealed container holds 0.020 moles of nitrogen (N2) gas, at a pressure of 1.5 atmospheres and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The mass density of the gas is closest to
A) 0.90 kg/m3.
B) 1.3 kg/m3.
C) 1.8 kg/m3.
D) 2.2 kg/m3.
E) 2.6 kg/m3.
A) 0.90 kg/m3.
B) 1.3 kg/m3.
C) 1.8 kg/m3.
D) 2.2 kg/m3.
E) 2.6 kg/m3.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
20
The figure shows a 50-kg frictionless cylindrical piston that floats on 0.68 mol of compressed air at 30°C. How far does the piston move if the temperature is increased to 300°C? 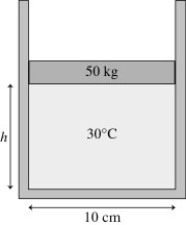
A) 120 cm
B) 250 cm
C) 130 cm
D) 1300 cm

A) 120 cm
B) 250 cm
C) 130 cm
D) 1300 cm

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
21
The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 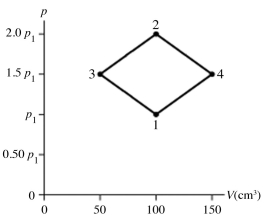
A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
22
A 25-L container holds ideal hydrogen (H2) gas at a gauge pressure of 0.25 atm and a temperature of 0°C. What mass of hydrogen gas is in this container? The ATOMIC mass of hydrogen is 1.0 g/mol, the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K, and 1.00 atm = 101 kPa.
A) 1.4 g
B) 2.8 g
C) 4.2 g
D) 5.6 g
E) 6.3 g
A) 1.4 g
B) 2.8 g
C) 4.2 g
D) 5.6 g
E) 6.3 g

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
23
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 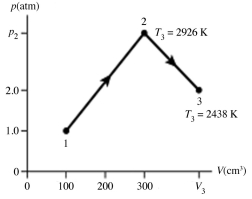
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
24
The interior of a refrigerator has a volume of 0.600 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator? The ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K.
A) 500 g
B) 560 g
C) 140 g
D) 270 g
E) 750 g
A) 500 g
B) 560 g
C) 140 g
D) 270 g
E) 750 g

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
25
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K.
A) 1.40 kg/m3
B) 1.00 kg/m3
C) 1.20 kg/m3
D) 1.60 kg/m3
E) 1.80 kg/m3
A) 1.40 kg/m3
B) 1.00 kg/m3
C) 1.20 kg/m3
D) 1.60 kg/m3
E) 1.80 kg/m3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
26
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 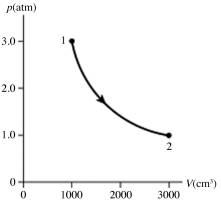
A) -160°C
B) 15°C
C) 390°C
D) 120°C

A) -160°C
B) 15°C
C) 390°C
D) 120°C

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of water (H2O) molecules are in a 4.00 m3 container at a pressure 8.00 × 105 N/m2 and temperature 600°C? The ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K.
A) 7.72 × 1026 mol
B) 641 mol
C) 441 mol
D) 3.86 × 1026 mol
E) 2.65 × 1026 mol
A) 7.72 × 1026 mol
B) 641 mol
C) 441 mol
D) 3.86 × 1026 mol
E) 2.65 × 1026 mol

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
28
The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 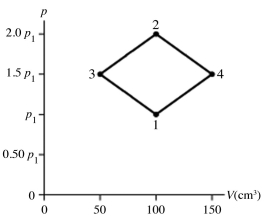
A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
29
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 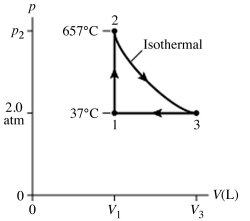
A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck


