Deck 17: Work, Heat, and the First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/98
Play
Full screen (f)
Deck 17: Work, Heat, and the First Law of Thermodynamics
1
An ideal gas is compressed in a well-insulated chamber using a well-insulated piston. This process is
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.
adiabatic.
2
When an ideal gas increases in volume at constant pressure, the average kinetic energy of the gas molecules
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.
A) increases.
B) decreases.
C) does not change.
D) may either increase or decrease, depending on whether or not the process is carried out adiabatically.
E) may or may not change, but insufficient information is given to make such a determination.
increases.
3
When a fixed amount of ideal gas goes through an isothermal expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.
its internal (thermal) energy does not change.
4
An ideal gas increases in temperature from 22°C to 42°C by two different processes. In one process, the temperature increases at constant volume, and in the other process the temperature increases at constant pressure. Which of the following statements about this gas are correct? (There may be more than one correct choice.)
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
5
When a fixed amount of ideal gas goes through an isobaric expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
6
The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 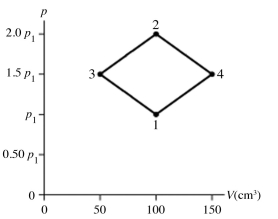
A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
7
The process shown in the T-V diagram in the figure is an 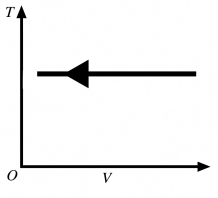
A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.

A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
8
When a vapor condenses
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
9
A container of ideal gas has a movable frictionless piston. This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water. Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
10
The process shown in the pV diagram in the figure is an 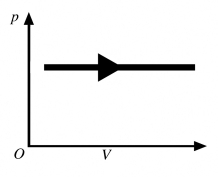
A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
11
When a fixed amount of ideal gas goes through an isochoric process
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
12
It is a well-known fact that water has a higher specific heat than iron. Now, consider equal masses of water and iron that are initially in thermal equilibrium. The same amount of heat, 30 calories, is added to each one. Which statement is true?
A) They remain in thermal equilibrium.
B) They are no longer in thermal equilibrium; the iron is warmer.
C) They are no longer in thermal equilibrium; the water is warmer.
D) It is impossible to say without knowing the exact mass involved.
E) It is impossible to say without knowing the exact specific heats.
A) They remain in thermal equilibrium.
B) They are no longer in thermal equilibrium; the iron is warmer.
C) They are no longer in thermal equilibrium; the water is warmer.
D) It is impossible to say without knowing the exact mass involved.
E) It is impossible to say without knowing the exact specific heats.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
13
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 
A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

A) 17 L
B) 69 L
C) 34 L
D) 8.6 L

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
14
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the aluminum and the copper are in thermal contact. The specific heat of aluminum is more than double that of copper. Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
A) The copper experiences a greater temperature change.
B) The aluminum experiences a greater temperature change.
C) Neither; both objects experience the same magnitude temperature change.
D) It is impossible to tell without knowing the masses.
E) It is impossible to tell without knowing the volumes.
A) The copper experiences a greater temperature change.
B) The aluminum experiences a greater temperature change.
C) Neither; both objects experience the same magnitude temperature change.
D) It is impossible to tell without knowing the masses.
E) It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
15
When a solid melts
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
16
The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 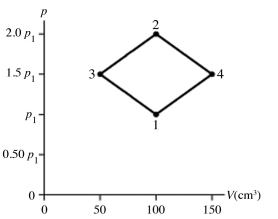
A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

A) -52°C, 390°C
B) 16°C, 47°C
C) 220°C, 660°C
D) 11°C, 32°C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
17
The process shown in the pV diagram in the figure is 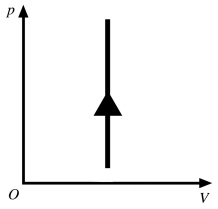
A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.

A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
18
When a gas undergoes an isothermal process, there is
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
19
A chunk of ice (T = -20°C) is added to a thermally insulated container of cold water (T = 0°C). What happens in the container?
A) The ice melts until thermal equilibrium is established.
B) The water cools down until thermal equilibrium is established.
C) Some of the water freezes and the chunk of ice gets larger.
D) None of the above things happen.
A) The ice melts until thermal equilibrium is established.
B) The water cools down until thermal equilibrium is established.
C) Some of the water freezes and the chunk of ice gets larger.
D) None of the above things happen.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
20
When a fixed amount of ideal gas goes through an adiabatic expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
21
A certain amount of ideal monatomic gas is maintained at constant volume as it is cooled from 455K to 405 K. This feat is accomplished by removing 400 J of heat from the gas. How much work is done by the gas during this process? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 0.00 J
B) 200 J
C) 400 J
D) -400 J
E) -200 J
A) 0.00 J
B) 200 J
C) 400 J
D) -400 J
E) -200 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
22
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 300 kPa, the initial volume is 0.020 m3, and the initial temperature is 390 K. The final pressure is 160kPa and the final temperature is 310 K. The change in the internal (thermal) energy of the gas is closest to
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
23
An adiabatic compression is performed on an ideal gas. The final pressure is equal to 0.560 times the initial pressure and the final volume equals 1.50 times the initial volume. What is the adiabatic constant for the gas?
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
24
During an isothermal process, 5.0 J of heat is removed from an ideal gas. How much work does the gas do during this process?
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
25
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.020 m3 to 0.040 m3. The final pressure is 120 kPa. The ideal gas constant is R = 8.314 J/mol ∙ K. The heat transfer to the gas is closest to
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.
A) 3.3 kJ.
B) 1.7 kJ.
C) -3.3 kJ.
D) -1.7 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
26
A steel container, equipped with a piston, contains 21 mol of an ideal gas at 465 K. The container is compressed isothermally to 90% of its original volume. How much work is done on the gas? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 8600 J
B) -73,300 J
C) -8500 J
D) 11 J
A) 8600 J
B) -73,300 J
C) -8500 J
D) 11 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
27
An ideal gas with γ = 1.67 is initially at 0°C in a volume of 10.0 L at a pressure of 1.00 atm. It is then expanded adiabatically to a volume of 10.4 L. What is the final temperature of the gas?
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
28
The temperature of an ideal gas in a sealed 0.40 m3 container is reduced from 400 K to 270 K. The final pressure of the gas is 30 kPA. The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The work done by the gas is closest to
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.
A) 0.00 kJ.
B) -19 kJ.
C) -25 kJ.
D) 19 kJ.
E) 25 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
29
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 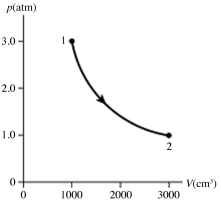
A) -160°C
B) 15°C
C) 390°C
D) 120°C

A) -160°C
B) 15°C
C) 390°C
D) 120°C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
30
The figure shows a pV diagram for 0.0066 mol of gas that undergoes the process 1 → 2 → 3. What is the pressure p2. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 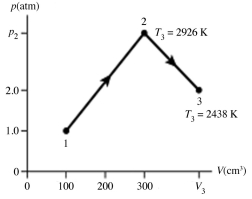
A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

A) 5.3 atm
B) 5.3 × 105 atm
C) 16 atm
D) 1.6 × 106 atm

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
31
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.03 m3 to 0.21 m3. The final pressure of the gas is 60 kPA The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.
A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
32
3.0 moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol∙K) and a molar heat capacity at constant pressure of 6.9 cal/(mol∙K) starts at 300 K and is heated at constant pressure to 320 K, then cooled at constant volume to its original temperature. How much heat flows into the gas during this two-step process?
A) 710 cal
B) -720 cal
C) 0.00 cal
D) 120 cal
E) -120 cal
A) 710 cal
B) -720 cal
C) 0.00 cal
D) 120 cal
E) -120 cal

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
33
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the change in internal (thermal) energy of the gas?
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
34
An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings. How much work is done by the gas if the outside pressure is slowly reduced, allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 645.0 Pa and a volume of 0.10 m3. The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J
A) 120 J
B) 390 J
C) -330 J
D) 6.0 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
35
The temperature of an ideal gas in a sealed 0.40-m3 rigid container is reduced from 350 K to 270 K. The final pressure of the gas is 60 kPA. The molar heat capacity at constant volume of the gas is 28.0 J/mol · K. The heat absorbed by the gas is closest to
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.
A) -24 kJ.
B) -31 kJ.
C) 24 kJ.
D) 31 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
36
An ideal monatomic gas cools from 455.0 K to 405.0 K at constant volume as 831 J of energy is removed from it. How many moles of gas are in the sample? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 2.50 mol
B) 2.15 mol
C) 1.50 mol
D) 1.33 mol
E) 0.725 mol
A) 2.50 mol
B) 2.15 mol
C) 1.50 mol
D) 1.33 mol
E) 0.725 mol

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
37
A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant volume. The same quantity of gas requires 900 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant pressure. What is the adiabatic constant γ for this gas?
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
38
How much work is done by 3.00 mol of ideal gas when it triples its volume at a constant temperature of 127°C? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 12.7 kJ
B) 9.97 kJ
C) 11.0 kJ
D) 15.3 kJ
E) 1.20 kJ
A) 12.7 kJ
B) 9.97 kJ
C) 11.0 kJ
D) 15.3 kJ
E) 1.20 kJ

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
39
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 300 kPa, the initial volume is 0.060 m3, and the initial temperature is 390 K. The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 150 kPa and the final temperature is 260 K. The work done by the gas is closest to
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.
A) 4500 J.
B) 2300 J.
C) 3400 J.
D) 5600 J.
E) 6800 J.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
40
A compression, at a constant pressure of 190 kPa, is performed on 5.0 moles of an ideal monatomic gas. The compression reduces the volume of the gas from 0.19 m3 to 0.12 m3. The ideal gas constant is R = 8.314 J/mol ∙ K. The work done by the gas is closest to
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.
A) -13 kJ.
B) 13 kJ.
C) -33 kJ.
D) 33 kJ.
E) 0.00 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
41
A cylinder contains 24.0 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.63 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol · K and the ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
A) -64 kJ.
B) -86 kJ.
C) 64 kJ.
D) 86 kJ.
E) -22 kJ.
A) -64 kJ.
B) -86 kJ.
C) 64 kJ.
D) 86 kJ.
E) -22 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
42
An ideal gas with γ = 1.30 occupies 7.0 L at 300 K and 200 kPa pressure. It is compressed adiabatically to 1/7 of its original volume, then cooled at constant volume to 300 K, and finally allowed to expand isothermally to 7.0 L. How much work does the gas do during this process? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K.
A) -980 J
B) 6400 J
C) -270,000 J
D) -6400 J
E) 980 J
A) -980 J
B) 6400 J
C) -270,000 J
D) -6400 J
E) 980 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
43
In an isochoric process, the internal (thermal) energy of an ideal gas decreases by 50 J. How much work does the gas do during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
44
If we use 67 W of power to heat 148 g of water, how long will it take to raise the temperature of the water from 15°C to 25°C? The specific heat of water is 4190 J/kg•K.
A) 93 s
B) 5.3 s
C) 22 s
D) 114 h
A) 93 s
B) 5.3 s
C) 22 s
D) 114 h

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
45
The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 9.00 x 104 Pa. If the volume of the system increases from 0.020 m3 to 0.050 m3, calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 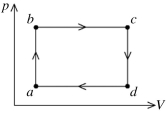


Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
46
A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 15.0°C to 37.0°C? (The specific heat for iron is 113 cal/kg•C°, 1 cal = 4.190 J)
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
47
In an isochoric process, the internal (thermal) energy of an ideal gas decreases by 50 J. How much heat is exchanged with the gas during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
48
A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 116°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 x 105 Pa on the gas. The gas is cooled until its temperature has decreased to 27°C For the gas 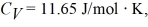
and the ideal gas constant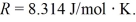
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?
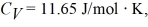
and the ideal gas constant
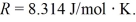
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
49
The gas in a perfectly insulated system does work at a rate of 13 W. At what rate is the internal (thermal) energy of the gas changing?
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
50
It is necessary to determine the specific heat of an unknown object. The mass of the object is measured to be 199.0 g. It is determined experimentally that it takes 16.0 J to raise the temperature 10.0°C. Find the specific heat of the object.
A) 8.04 J/kg ∙ K
B) 1600 J/kg ∙ K
C) 0.00120 J/kg ∙ K
D) 3.18 × 106 J/kg ∙ K
A) 8.04 J/kg ∙ K
B) 1600 J/kg ∙ K
C) 0.00120 J/kg ∙ K
D) 3.18 × 106 J/kg ∙ K

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
51
A fixed amount of ideal gas goes through a process abc. In state a, the temperature of the gas is 152°C, its pressure is 1.25 atm, and it occupies a volume of 0.250 m3. It then undergoes an isothermal expansion to state b that doubles its volume, followed by an isobaric compression back to its original volume at state c. (Hint: First show this process on a pV diagram.) The ideal gas constant is 8.314 J/mol ∙ K, and 1.00 atm = 1.01 × 105 Pa.
(a) How many moles does this gas contain?
(b) What is the change in the internal energy of the gas between states a and b?
(c) What is the net work done on (or by) this gas during the entire process?
(d) What is the temperature of the gas in state c?
(a) How many moles does this gas contain?
(b) What is the change in the internal energy of the gas between states a and b?
(c) What is the net work done on (or by) this gas during the entire process?
(d) What is the temperature of the gas in state c?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
52
During an adiabatic process, 20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa. The ideal gas constant is R = 8.314 J/mol ∙ K.
(a) What is the final volume of the gas?
(b) How much heat does the gas exchange during this process?
(c) What is the change in the internal (thermal) energy of the gas during this process?
(a) What is the final volume of the gas?
(b) How much heat does the gas exchange during this process?
(c) What is the change in the internal (thermal) energy of the gas during this process?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
53
A cylinder contains 23 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.43 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol · K and the ideal gas constant is R = 8.314 J/mol ∙ K. The heat absorbed by the gas is closest to
A) -130 kJ.
B) -94 kJ.
C) 130 kJ.
D) 94 kJ.
E) -33 kJ.
A) -130 kJ.
B) -94 kJ.
C) 130 kJ.
D) 94 kJ.
E) -33 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
54
An ideal gas initially at 300 K and occupying a volume of 20 L is adiabatically compressed. If its final temperature is 400 K and γ = 1.30, what is its final volume?
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
55
A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W. At what rate is the internal (thermal) energy of the gas changing?
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
56
An ideal gas is allowed to expand slowly at constant temperature to twice its original volume. During the expansion, the gas absorbs 200 kJ of heat.
(a) What is the change in the internal (thermal) energy of the gas during the expansion?
(b) How much work does the gas do during the expansion?
(a) What is the change in the internal (thermal) energy of the gas during the expansion?
(b) How much work does the gas do during the expansion?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
57
In a thermodynamic process involving 7.8 moles of an ideal gas, the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3. The gas expands adiabatically to a volume of 0.080 m3. For this gas, CV = 12.27 J/mol · K, and the ideal gas constant is R = 8.314 J/mol ∙ K. Calculate the work done by the gas during this expansion.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
58
A container with rigid walls is filled with 4 mol of air with CV = 2.5R. How much does the internal (thermal) energy change if the temperature of the air rises from 16°C to 437°C? The ideal gas constant is R = 8.314 J/mol ∙ K.
A) 35 kJ
B) 421 J
C) 3.5 kJ
D) 8.75 kJ
A) 35 kJ
B) 421 J
C) 3.5 kJ
D) 8.75 kJ

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
59
The pV diagram shown is for 7.50 moles of an ideal diatomic gas taken through a cycle from a to b to c. The ideal gas constant is R = 8.314 J/mol ∙ K. 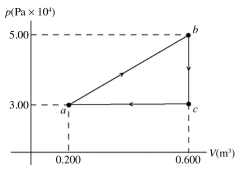
(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?

(a) What is the highest temperature reached by the gas during the cycle?
(b) What net work does the gas do during the cycle?
(c) How much heat is exchanged with the gas during part bc of the cycle? Does it enter or leave the gas?
(d) What is the change in the internal (thermal) energy of the gas during part bc of the cycle?
(e) What is the change in the internal (thermal) energy of the gas during the entire cycle?

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
60
During an adiabatic process, an ideal gas does 25 J of work. What is the change in the internal (thermal) energy of the gas during this process?
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
61
How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 87°C to produce water at a final temperature of 10.0°C? Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg·C° and of ice is 2050 J/kg·C°. For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
62
Heat is added to a 2.0 kg piece of ice at a rate of 793.0 kW. How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
63
An 80-g aluminum calorimeter contains 380 g of water at an equilibrium temperature of 20°C. A 120-g piece of metal, initially at 352°C, is added to the calorimeter. The final temperature at equilibrium is 32°C. Assume there is no external heat exchange. The specific heats of aluminum and water are 910 J/kg·K and 4190 J/kg·K, respectively. The specific heat of the metal is closest to
A) 520 J/kg ∙ K.
B) 480 J/kg ∙ K.
C) 390 J/kg ∙ K.
D) 350 J/kg ∙ K.
E) 560 J/kg ∙ K.
A) 520 J/kg ∙ K.
B) 480 J/kg ∙ K.
C) 390 J/kg ∙ K.
D) 350 J/kg ∙ K.
E) 560 J/kg ∙ K.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
64
A substance has a melting point of 20°C and a heat of fusion of 3.9 x 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.8 x 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K), 1000 J/(kg∙K), and 400 J/(kg∙K), respectively. The quantity of heat required to raise the temperature of 3.80 kg of the substance from -61°C to 128°C, at a pressure of 1.0 atm, is closest to
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.
A) 620 kJ.
B) 470 kJ.
C) 560 kJ.
D) 210 kJ.
E) 770 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
65
If 2.0 g of water at 0.00°C is to be vaporized, how much heat must be added to it? The specific heat of water is 1.0 cal/g∙K, its heat of fusion is 80 cal/g, and its heat of vaporization is 539 cal/g.
A) 1100 cal
B) 1100 kcal
C) 1200 cal
D) 1300 cal
E) 1500 cal
A) 1100 cal
B) 1100 kcal
C) 1200 cal
D) 1300 cal
E) 1500 cal

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
66
What is the steady state rate of heat flow through a pane of glass that is 40.0 cm by 30.0 cm with a thickness of 4.00 mm when the outside temperature of the glass is -10.0°C and its inside temperature is 25.0°C? The thermal conductivity of glass is 0.105 W/(m∙K), the specific heat of glass is 0.180 cal/(g∙°C), and 1 cal = 4.190 J.
A) 24.2 W
B) 3.81 W
C) 18.6 W
D) 47.3 W
E) 110 W
A) 24.2 W
B) 3.81 W
C) 18.6 W
D) 47.3 W
E) 110 W

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
67
If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 106 J/kg and its specific heat is is 4190 J/(kg∙K).
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
68
Two experimental runs are performed to determine the calorimetric properties of an alcohol that has a melting point of -10°C. In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5.0°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C. The specific heat of water is 4190 J/kg·K. Assume that no heat is exchanged with the styrofoam container and with the surroundings. The heat of fusion of the alcohol is closest to
A) 5.5 × 104 J/kg
B) 6.3 × 104 J/kg
C) 7.1 × 104 J/kg
D) 7.9 × 104 J/kg
E) 8.7 × 104 J/kg
A) 5.5 × 104 J/kg
B) 6.3 × 104 J/kg
C) 7.1 × 104 J/kg
D) 7.9 × 104 J/kg
E) 8.7 × 104 J/kg

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
69
A 406.0 kg copper bar is put into a smelter for melting. The initial temperature of the copper is 300.0 K. How much heat must the smelter produce to completely melt the copper bar? (The specific heat for copper is 386 J/kg•K, the heat of fusion for copper is 205 kJ/kg, and its melting point is 1357 K.)
A) 2.49 × 105 kJ
B) 1.66 × 1011 kJ
C) 1.66 × 108 kJ
D) 2.96 × 105 kJ
A) 2.49 × 105 kJ
B) 1.66 × 1011 kJ
C) 1.66 × 108 kJ
D) 2.96 × 105 kJ

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
70
A copper cylinder with a mass of 125 g and temperature of 345°C is cooled by dropping it into a glass beaker containing 565 g of water initially at 20.0°C. The mass of the beaker is 50.0 g and the specific heat of the glass is 840 J/kg∙K. What is the final equilibrium temperature of the system, assuming the cooling takes place very quickly, so that no energy is lost to the air? The specific heat of copper is 385 J/kg∙K and that of water is 4190 J/kg∙K.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
71
A person pours 330 g of water at 45°C into an 855-g aluminum container with an initial temperature of 10°C. The specific heat of aluminum is 900 J/(kg∙K) and that of water is 4190 J/(kg∙K). What is the final temperature of the system, assuming no heat is exchanged with the surroundings?
A) 28°C
B) 32°C
C) 31°C
D) 33°C
E) 35°C
A) 28°C
B) 32°C
C) 31°C
D) 33°C
E) 35°C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
72
Heat is added to a pure substance in a closed container at a constant rate. The figure shows a graph of the temperature of the substance as a function of time. If Lf = latent heat of fusion and Lv = latent heat of vaporization, what is the value of the ratio Lv / Lf for this substance? 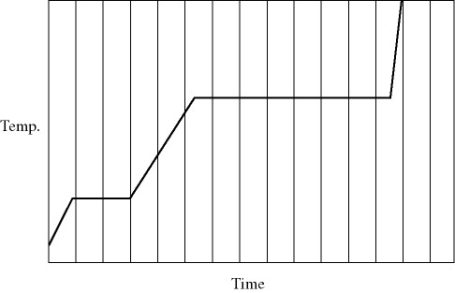
A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5

A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
73
A block of ice at 0.000°C is added to a well-insulated 147-g aluminum calorimeter cup that holds 200 g of water at 10.0°C. The water and aluminum cup are in thermal equilibrium, and the specific heat of aluminum is 910 J/(kg∙K). If all but 2.00 g of ice melt, what was the original mass of the block of ice? The specific heat of water is 4190 J/(kg∙K), its latent heat of fusion is 334 kJ/kg, and its latent heat of vaporization is 2260 kJ/kg.
A) 31.1 g
B) 35.6 g
C) 38.8 g
D) 42.0 g
E) 47.6 g
A) 31.1 g
B) 35.6 g
C) 38.8 g
D) 42.0 g
E) 47.6 g

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
74
A 400-g piece of metal at 120.0°C is dropped into a cup containing 450 g of water at 15.0°C. The final temperature of the system is measured to be 40.0°C. What is the specific heat of the metal, assuming no heat is exchanged with the surroundings or the cup? The specific heat of water is 4190 J/(kg∙K).
A) 1470 J/(kg ∙ K)
B) 2830 J/(kg ∙ K)
C) 3420 J/(kg ∙ K)
D) 3780 J/(kg ∙ K)
E) 4280 J/(kg ∙ K)
A) 1470 J/(kg ∙ K)
B) 2830 J/(kg ∙ K)
C) 3420 J/(kg ∙ K)
D) 3780 J/(kg ∙ K)
E) 4280 J/(kg ∙ K)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
75
Two experimental runs are performed to determine the calorimetric properties of an alcohol that has a melting point of -10°C. In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5.0°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C. The specific heat of water is 4190 J/kg·K. Assume that no heat is exchanged with the styrofoam container and with the surroundings. The specific heat of the alcohol is closest to
A) 1700 J/kg·K.
B) 1900 J/kg·K.
C) 2100 J/kg·K.
D) 2300 J/kg·K.
E) 2500 J/kg·K.
A) 1700 J/kg·K.
B) 1900 J/kg·K.
C) 2100 J/kg·K.
D) 2300 J/kg·K.
E) 2500 J/kg·K.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
76
A person makes ice tea by adding ice to 1.8 kg of hot tea, initially at 80°C. How many kilograms of ice, initially at 0.00°C, are required to bring the mixture to 10°C? The heat of fusion of ice is 334 kJ/kg, and we can assume that tea has essentially the same thermal properties as water, so its specific heat is 4190 J/(kg∙K).
A) 1.0 kg
B) 1.2 kg
C) 1.4 kg
D) 1.5 kg
E) 1.7 kg
A) 1.0 kg
B) 1.2 kg
C) 1.4 kg
D) 1.5 kg
E) 1.7 kg

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
77
A 905-g meteor impacts the earth at a speed of 1629 m/s. If all of its energy is entirely converted to heat in the meteor, what will be the resulting temperature rise of the meteor, assuming it does not melt? The specific heat for the meteor material is 472 J/kg∙K, which is about the same as that of iron.
A) 2810°C
B) 2,540,000°C
C) 3.10°C
D) 11,700°C
A) 2810°C
B) 2,540,000°C
C) 3.10°C
D) 11,700°C

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
78
Under steady state conditions, a piece of wood 350 mm by 350 mm and 15 mm thick conducts heat through its thickness and loses no appreciable heat through its well-insulated sides. The rate of heat flow is measured to be 14.0 W when the temperature difference across its thickness is 28 C°. Determine the thermal conductivity of this wood.
A) 9.2 × 10-4 W/(m∙C°)
B) 270 W/(m∙C°)
C) 16 W/(m∙C°)
D) 0.061 W/(m∙C°)
E) 33 W/(m∙C°)
A) 9.2 × 10-4 W/(m∙C°)
B) 270 W/(m∙C°)
C) 16 W/(m∙C°)
D) 0.061 W/(m∙C°)
E) 33 W/(m∙C°)

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
79
A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 22.00°C. A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A) 3850 J/kg ∙ K.
B) 2730 J/kg ∙ K.
C) 4450 J/kg ∙ K.
D) 4950 J/kg ∙ K.
E) 5450 J/kg ∙ K.
A) 3850 J/kg ∙ K.
B) 2730 J/kg ∙ K.
C) 4450 J/kg ∙ K.
D) 4950 J/kg ∙ K.
E) 5450 J/kg ∙ K.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck
80
A substance has a melting point of 20°C and a heat of fusion of 3.5 × 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.0 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K), 1000 J/(kg∙K), and 400 J/(kg∙K), respectively. The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C, at a pressure of 1.0 atmosphere, is closest to
A) 70 kJ.
B) 14 kJ.
C) 21 kJ.
D) 30 kJ.
E) 44 kJ.
A) 70 kJ.
B) 14 kJ.
C) 21 kJ.
D) 30 kJ.
E) 44 kJ.

Unlock Deck
Unlock for access to all 98 flashcards in this deck.
Unlock Deck
k this deck


