Exam 17: Work, Heat, and the First Law of Thermodynamics
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion31 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Impulse and Momentum20 Questions
Exam 10: Energy43 Questions
Exam 11: Work100 Questions
Exam 12: Rotation of a Rigid Body113 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Oscillations49 Questions
Exam 15: Fluids and Elasticity72 Questions
Exam 16: A Macroscopic Description of Matter29 Questions
Exam 17: Work, Heat, and the First Law of Thermodynamics98 Questions
Exam 18: The Micromacro Connection39 Questions
Exam 19: Heat Engines and Refrigerators50 Questions
Exam 20: Traveling Waves49 Questions
Exam 21: Superpositions64 Questions
Exam 22: Wave Optics51 Questions
Exam 23: Ray Optics63 Questions
Exam 24: Optical Instruments49 Questions
Exam 25: Electric Charges and Forces26 Questions
Exam 26: The Electric Field32 Questions
Exam 27: Gausss Law41 Questions
Exam 28: The Electric Potential40 Questions
Exam 29: Potential and Field57 Questions
Exam 30: Current and Resistance32 Questions
Exam 31: Fundamentals of Circuits68 Questions
Exam 32: The Magnetic Field87 Questions
Exam 33: Electromagnetic Induction66 Questions
Exam 34: Electromagnetic Fields and Waves52 Questions
Exam 35: Ac Circuits46 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics8 Questions
Exam 38: Quantization54 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics39 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
Heat is added to a 2.0 kg piece of ice at a rate of 793.0 kW. How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 106 J/kg and its specific heat is is 4190 J/(kg∙K).
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
A block of ice at 0.000°C is added to a well-insulated 147-g aluminum calorimeter cup that holds 200 g of water at 10.0°C. The water and aluminum cup are in thermal equilibrium, and the specific heat of aluminum is 910 J/(kg∙K). If all but 2.00 g of ice melt, what was the original mass of the block of ice? The specific heat of water is 4190 J/(kg∙K), its latent heat of fusion is 334 kJ/kg, and its latent heat of vaporization is 2260 kJ/kg.
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
A
A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 22.00°C. A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
(Multiple Choice)
4.9/5  (30)
(30)
Some properties of glass are listed here.
Density: 2300 kg/m3
Specific heat: 840 J/kg·C°
Coefficient of linear thermal expansion: 8.5 × 10-6 (C°)-1
Thermal conductivity: 0.80 W/(m·C°)
A glass window pane is 2.7 m high, 2.4 m wide, and 2.0 mm thick. The temperature at the inner surface of the glass is 22°C and at the outer surface 4.0°C. How much heat is lost each hour through the window under steady state conditions?
(Multiple Choice)
4.9/5  (31)
(31)
During an isothermal process, 5.0 J of heat is removed from an ideal gas. How much work does the gas do during this process?
(Multiple Choice)
4.8/5  (40)
(40)
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 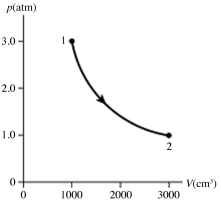
(Multiple Choice)
4.7/5  (43)
(43)
A container of ideal gas has a movable frictionless piston. This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water. Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
(Multiple Choice)
4.7/5  (36)
(36)
A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W. At what rate is the internal (thermal) energy of the gas changing?
(Multiple Choice)
4.8/5  (40)
(40)
A 905-g meteor impacts the earth at a speed of 1629 m/s. If all of its energy is entirely converted to heat in the meteor, what will be the resulting temperature rise of the meteor, assuming it does not melt? The specific heat for the meteor material is 472 J/kg∙K, which is about the same as that of iron.
(Multiple Choice)
4.8/5  (34)
(34)
Betelgeuse is a red supergiant star in the constellation Orion. It radiates heat at the rate of 2.70 × 1030 W and has a surface temperature of 3000 K. Assuming that it is a perfect emitter, what is the radius of Betelgeuse? The Stefan-Boltzmann constant is 5.670 × 10-8 W/m2 · K4.
(Multiple Choice)
4.8/5  (36)
(36)
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram. The initial pressure is 300 kPa, the initial volume is 0.060 m3, and the initial temperature is 390 K. The ideal gas constant is R = 8.314 J/mol ∙ K. The final pressure is 150 kPa and the final temperature is 260 K. The work done by the gas is closest to
(Multiple Choice)
4.8/5  (28)
(28)
A 406.0 kg copper bar is put into a smelter for melting. The initial temperature of the copper is 300.0 K. How much heat must the smelter produce to completely melt the copper bar? (The specific heat for copper is 386 J/kg•K, the heat of fusion for copper is 205 kJ/kg, and its melting point is 1357 K.)
(Multiple Choice)
4.9/5  (35)
(35)
A radiating body originally has a Kelvin temperature To, and its surroundings are at 500K (To > 500K). If the Kelvin temperature of the radiating body is increased to 3To, the net rate at which the body radiates increases by a factor of 333. What was the original temperature To?
(Short Answer)
4.9/5  (41)
(41)
A 400-g piece of metal at 120.0°C is dropped into a cup containing 450 g of water at 15.0°C. The final temperature of the system is measured to be 40.0°C. What is the specific heat of the metal, assuming no heat is exchanged with the surroundings or the cup? The specific heat of water is 4190 J/(kg∙K).
(Multiple Choice)
4.8/5  (32)
(32)
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.03 m3 to 0.21 m3. The final pressure of the gas is 60 kPA The ideal gas constant is R = 8.314 J/mol ∙ K. The change in the internal (thermal) energy of the gas is closest to
(Multiple Choice)
4.8/5  (32)
(32)
A cube at 100.0°C radiates heat at a rate of 80.0 J/s. If the length of each side is cut in half, the rate at which it will now radiate is closest to
(Multiple Choice)
4.7/5  (25)
(25)
A cube at 100°C radiates heat at a rate of 80.0 J/s. If its surface temperature is increased to 200°C, the rate at which it will now radiate is closest to
(Multiple Choice)
4.9/5  (38)
(38)
A chunk of ice (T = -20°C) is added to a thermally insulated container of cold water (T = 0°C). What happens in the container?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)