Deck 6: An Overview of Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/42
Play
Full screen (f)
Deck 6: An Overview of Organic Reactions
1
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an elimination reaction.
D)an addition reaction.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an elimination reaction.
D)an addition reaction.
a substitution reaction.
2
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.Using the bond dissociations values in the table below,calculate the ΔH° for the reaction in Diagram 2.Show your calculations for full credit.
Bond
D (kJ/mol)
(CH3)3C−Br
263
(CH3)3C−OH
380
HO−H
498
H−Br
366
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.Using the bond dissociations values in the table below,calculate the ΔH° for the reaction in Diagram 2.Show your calculations for full credit.
Bond
D (kJ/mol)
(CH3)3C−Br
263
(CH3)3C−OH
380
HO−H
498
H−Br
366
ΔH°
= ΔH° bonds broken − ΔH° bonds formed
= 761 kJ/mol − 746 kJ/mol
= +15 kJ/mol
Bonds Broken
Bonds Formed
(CH3)3C−Br
263 kJ/mol
(CH3)3C−OH
380 kJ/mol
HO−H
498 kJ/mol
H−Br
366 kJ/mol
761 kJ/mol
746 kJ/mol
= ΔH° bonds broken − ΔH° bonds formed
= 761 kJ/mol − 746 kJ/mol
= +15 kJ/mol
Bonds Broken
Bonds Formed
(CH3)3C−Br
263 kJ/mol
(CH3)3C−OH
380 kJ/mol
HO−H
498 kJ/mol
H−Br
366 kJ/mol
761 kJ/mol
746 kJ/mol
3
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 2,label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 2,label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.
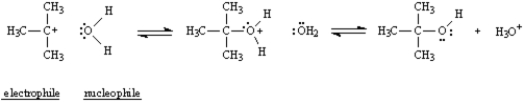
4
Refer to Exhibit 6-7.Draw a qualitative energy diagram for the reaction (assume that the first step is slower than the second step).Label fully.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
5
_____ A reaction where ΔG° is negative.
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
6
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 1,add curved arrows to indicate electron flow.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 1,add curved arrows to indicate electron flow.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
7
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).
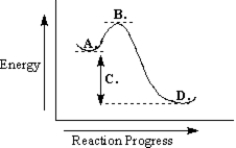
Refer to Exhibit 6-9.The free-energy change for the reaction is indicated at point _____ on the diagram.
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The free-energy change for the reaction is indicated at point _____ on the diagram.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
8
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The products are found at point _____ on the diagram.
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The products are found at point _____ on the diagram.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
9
Exhibit 6-7
Consider this reaction when answering the following question(s):
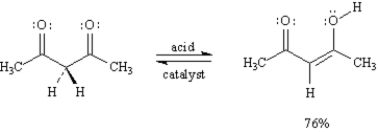
Refer to Exhibit 6-7.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an addition reaction.
D)an elimination reaction.
Consider this reaction when answering the following question(s):

Refer to Exhibit 6-7.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an addition reaction.
D)an elimination reaction.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
10
The original question has been combined with #21 as part
b.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
b.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
11
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 1,Species B is:
A)a carbene
B)a carbanion
C)a carbocation
D)a radical
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 1,Species B is:
A)a carbene
B)a carbanion
C)a carbocation
D)a radical

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
12
Exhibit 6-7
Consider this reaction when answering the following question(s):

Refer to Exhibit 6-7.
A)The structures below show the step-wise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.
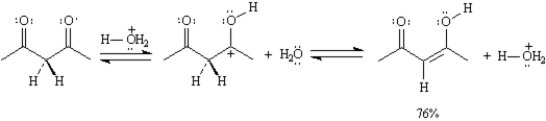
B)Calculate Keq for the reaction in part
C)Calculate ΔG° for the reaction in part
Consider this reaction when answering the following question(s):

Refer to Exhibit 6-7.
A)The structures below show the step-wise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.

B)Calculate Keq for the reaction in part
C)Calculate ΔG° for the reaction in part

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
13
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The transition state is found at point _____ on the diagram.
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The transition state is found at point _____ on the diagram.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
14
_____ ΔG° = −RT ln Keq
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
15
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.The reaction in Diagram 2 is an example of:
A)a pericyclic reaction.
B)a radical reaction.
C)a concerted reaction.
D)a stepwise reaction.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.The reaction in Diagram 2 is an example of:
A)a pericyclic reaction.
B)a radical reaction.
C)a concerted reaction.
D)a stepwise reaction.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
16
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The reactants are found at point _____ on the diagram.
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The reactants are found at point _____ on the diagram.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
17
_____ The energy needed by reactants to reach the transition state.
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
18
_____ A species that lies at an energy minimum between steps on a reaction.
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate
A)transition state
B)endergonic reaction
C)activation energy
D)standard Gibbs free energy change
E)exergonic reaction
F)reaction intermediate

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
19
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The reaction depicted in this reaction energy diagram can best be described as:
A)a slow exothermic reaction
B)a fast exothermic reaction
C)a slow endothermic reaction
D)a fast endothermic reaction
Use the reaction energy diagram below to answer the following question(s).

Refer to Exhibit 6-9.The reaction depicted in this reaction energy diagram can best be described as:
A)a slow exothermic reaction
B)a fast exothermic reaction
C)a slow endothermic reaction
D)a fast endothermic reaction

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
20
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 2,draw arrows on the structures showing electron flow.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.In Diagram 2,draw arrows on the structures showing electron flow.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
21
The alkane formed by hydrogenation of (S)-4-methyl-1-hexene is optically active while the one formed by hydrogenation of (S)-3-methyl-1-pentene is not.Explain.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following process. 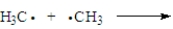 Which of the following correctly applies?
Which of the following correctly applies?
A)termination step
B)unsymmetrical bond formation
C)polar reaction
D)produces a radical
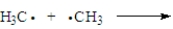 Which of the following correctly applies?
Which of the following correctly applies?A)termination step
B)unsymmetrical bond formation
C)polar reaction
D)produces a radical

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following could act as a nucleophile?
A)HCl
B)CH3NH2
C)BF3
D)CH3Br
A)HCl
B)CH3NH2
C)BF3
D)CH3Br

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following could act as an electrophile?
A)CN-
B)NH2-
C)NH3
D)H3O+
A)CN-
B)NH2-
C)NH3
D)H3O+

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following grayscale electrostatic potential map.The regions are labeled as to color. 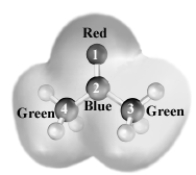 Which atom is the most likely to be attacked by a nucleophile?
Which atom is the most likely to be attacked by a nucleophile?
A)1
B)2
C)3
D)4
 Which atom is the most likely to be attacked by a nucleophile?
Which atom is the most likely to be attacked by a nucleophile?A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
26
Write the mechanism of the reaction of trans-2-butene with hydrogen bromide.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
27
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.Based on all of the data presented in Exhibit 6-10,draw a qualitative reaction energy diagram for the reaction of 2-bromo-2-methylpropane with water.The first step is the slowest step of the reaction.Fully label the diagram,including the coordinates.
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.

Refer to Exhibit 6-10.Based on all of the data presented in Exhibit 6-10,draw a qualitative reaction energy diagram for the reaction of 2-bromo-2-methylpropane with water.The first step is the slowest step of the reaction.Fully label the diagram,including the coordinates.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
28
Consider a reaction with the following thermodynamic properties. ΔH° = 77.7 kJ
ΔS° = -35.7 J/(K • mol)
ΔG°= 88.4 kJ
This reaction:
A)will be spontaneous at low temperatures.
B)has bonds in the products that are weaker than the reactants.
C)may have fewer and more complicated molecules in the product.
D)will proceed very slowly.
ΔS° = -35.7 J/(K • mol)
ΔG°= 88.4 kJ
This reaction:
A)will be spontaneous at low temperatures.
B)has bonds in the products that are weaker than the reactants.
C)may have fewer and more complicated molecules in the product.
D)will proceed very slowly.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
29
A reaction that establishes equilibrium with almost no reactants present:
A)is exergonic.
B)has a negative ΔG°.
C)has a large Keq.
D)all of the above.
A)is exergonic.
B)has a negative ΔG°.
C)has a large Keq.
D)all of the above.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following energy diagram for an enzyme-catalyzed reaction. 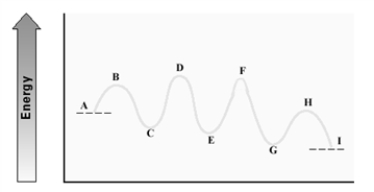 Which step is probably the slowest?
Which step is probably the slowest?
A)A - C
B)C - E
C)E - G
D)G - I
 Which step is probably the slowest?
Which step is probably the slowest?A)A - C
B)C - E
C)E - G
D)G - I

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
31
In a polar reaction mechanism,the atom that gives away electrons in a neutral nucleophile will end up as a(n):
A)cation
B)anion
C)radical
D)neutral molecule
A)cation
B)anion
C)radical
D)neutral molecule

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
32
The following represents the carbocation intermediate in the reaction of an alkene with HBr. 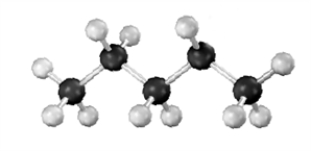 Draw the skeletal structure of the possible products and reactants.
Draw the skeletal structure of the possible products and reactants.
 Draw the skeletal structure of the possible products and reactants.
Draw the skeletal structure of the possible products and reactants.
Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following grayscale electrostatic potential map.The regions are labeled as to color. 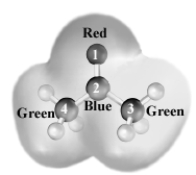 Which atom is the most electron poor?
Which atom is the most electron poor?
A)1
B)2
C)3
D)4
 Which atom is the most electron poor?
Which atom is the most electron poor?A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
34
What are the major differences between a reaction occurring in flask in the laboratory compared to a reaction occurring in a biological system?

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following energy diagram. 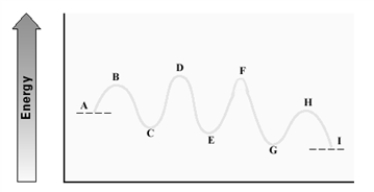 Which step has the least endergonic ΔG±?
Which step has the least endergonic ΔG±?
A)A - C
B)C - E
C)E - G
D)G - I
 Which step has the least endergonic ΔG±?
Which step has the least endergonic ΔG±?A)A - C
B)C - E
C)E - G
D)G - I

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following compounds would produce a single product when treated with Cl2 in the presence of ultraviolet radiation?
A)butane
B)2-methylpropane
C)2,2,-dimethylpropane
D)All would produce multiple products.
A)butane
B)2-methylpropane
C)2,2,-dimethylpropane
D)All would produce multiple products.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following energy diagram for an enzyme-catalyzed reaction. 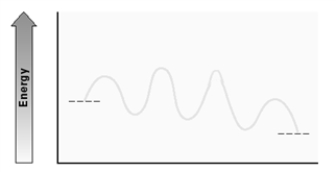 How many transition states are involved?
How many transition states are involved?
A)2
B)3
C)4
D)5
 How many transition states are involved?
How many transition states are involved?A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
38
The enzyme aconitase catalyzes the hydration of the alkene functional group of aconitic acid to give two products,citric acid and isocitric acid.Isocitric acid is optically active;citric acid is not optically active.Based on your knowledge of alkene hydration and optical activity,the structure of citric acid is: 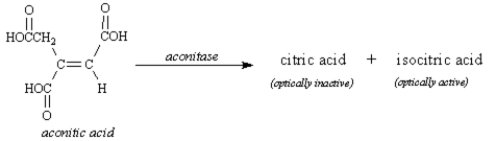
A)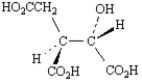
B)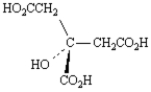
C)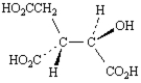
D)cannot be determined

A)

B)

C)

D)cannot be determined

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
39
A reaction has ΔH° = -14.7 kJ and ΔS° of 35.7 J/(K • mol),will
A)be exergonic.
B)have a positive ΔG°.
C)have a very small Keq.
D)will proceed rapidly.
A)be exergonic.
B)have a positive ΔG°.
C)have a very small Keq.
D)will proceed rapidly.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the following energy diagram for an enzyme-catalyzed reaction. 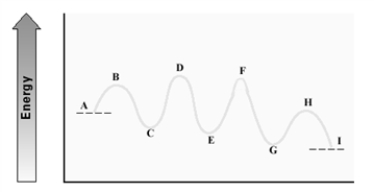 Which step has the least energetic transition state?
Which step has the least energetic transition state?
A)A - C
B)C - E
C)E - G
D)G - I
 Which step has the least energetic transition state?
Which step has the least energetic transition state?A)A - C
B)C - E
C)E - G
D)G - I

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
41
Write the mechanism of the reaction between hydroxide ion and methyl chloride.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
42
Write the mechanism of the reaction between water and ammonia.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck


