Exam 6: An Overview of Organic Reactions
Exam 1: Structure and Bonding29 Questions
Exam 2: Polar Covalent Bonds;acids and Bases50 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry37 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry37 Questions
Exam 5: Stereochemistry at Tetrahedral Centers43 Questions
Exam 6: An Overview of Organic Reactions42 Questions
Exam 6: Par 12 Questions
Exam 7: Alkenes: Structure and Reactivity37 Questions
Exam 8: Alkenes: Reactions and Synthesis42 Questions
Exam 9: Alkynes: an Introduction to Organic Synthesis31 Questions
Exam 10: Organohalides31 Questions
Exam 11: Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations34 Questions
Exam 11: Par 22 Questions
Exam 12: Structure Determination: Mass Spectrometry and Infrared Spectroscopy42 Questions
Exam 13: Structure Determination: Nuclear Magnetic Resonance Spectroscopy46 Questions
Exam 14: Conjugated Compounds and Ultraviolet Spectroscopy40 Questions
Exam 14: Par 32 Questions
Exam 15: Benzene and Aromaticity47 Questions
Exam 16: Chemistry of Benzene: Electrophilic Aromatic Substitution30 Questions
Exam 17: Alcohols and Phenols44 Questions
Exam 18: Ethers and Epoxides;thiols and Sulfides33 Questions
Exam 19: Aldehydes and Ketones: Nucleophilic Addition Reactions48 Questions
Exam 19: Par 42 Questions
Exam 20: Carboxylic Acids and Nitriles32 Questions
Exam 21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions44 Questions
Exam 22: Carbonyl Alpha-Substitution Reactions33 Questions
Exam 23: Carbonyl Condensation Reactions36 Questions
Exam 23: Par 52 Questions
Exam 24: Amines and Heterocycles41 Questions
Exam 25: Biomolecules: Carbohydrates63 Questions
Exam 26: Biomolecules: Amino Acids,peptides,and Proteins45 Questions
Exam 27: Par 72 Questions
Exam 27: Biomolecules: Lipids54 Questions
Exam 28: Biomolecules: Nucleic Acids44 Questions
Exam 29: The Organic Chemistry of Metabolic Pathways48 Questions
Exam 30: Orbitals and Organic Chemistry: Pericyclic Reactions44 Questions
Exam 31: Synthetic Polymers33 Questions
Exam 30: Par 12 Questions
Select questions type
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).
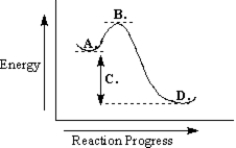 -Refer to Exhibit 6-9.The transition state is found at point _____ on the diagram.
-Refer to Exhibit 6-9.The transition state is found at point _____ on the diagram.
Free
(Short Answer)
4.9/5  (36)
(36)
Correct Answer:
B
Consider the following energy diagram for an enzyme-catalyzed reaction. 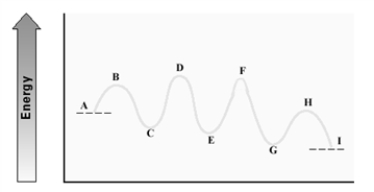 Which step is probably the slowest?
Which step is probably the slowest?
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
C
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
 -Refer to Exhibit 6-10.In Diagram 1,Species B is:
-Refer to Exhibit 6-10.In Diagram 1,Species B is:
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
C
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).
 -Refer to Exhibit 6-9.The products are found at point _____ on the diagram.
-Refer to Exhibit 6-9.The products are found at point _____ on the diagram.
(Short Answer)
4.8/5  (38)
(38)
_____ The energy needed by reactants to reach the transition state.
(Multiple Choice)
4.9/5  (37)
(37)
The alkane formed by hydrogenation of (S)-4-methyl-1-hexene is optically active while the one formed by hydrogenation of (S)-3-methyl-1-pentene is not.Explain.
(Essay)
4.9/5  (38)
(38)
Write the mechanism of the reaction between hydroxide ion and methyl chloride.
(Essay)
4.8/5  (41)
(41)
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
 -Refer to Exhibit 6-10.Based on all of the data presented in Exhibit 6-10,draw a qualitative reaction energy diagram for the reaction of 2-bromo-2-methylpropane with water.The first step is the slowest step of the reaction.Fully label the diagram,including the coordinates.
-Refer to Exhibit 6-10.Based on all of the data presented in Exhibit 6-10,draw a qualitative reaction energy diagram for the reaction of 2-bromo-2-methylpropane with water.The first step is the slowest step of the reaction.Fully label the diagram,including the coordinates.
(Essay)
4.9/5  (35)
(35)
Consider the following energy diagram for an enzyme-catalyzed reaction. 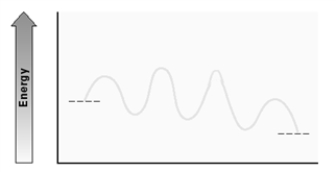 How many transition states are involved?
How many transition states are involved?
(Multiple Choice)
4.8/5  (38)
(38)
The original question has been combined with #21 as part
b.This placeholder question is here to maintain the integrity of the numbering system between the printed copy and ExamView.Therefore,it has been marked "do not use on test" in ExamView's question information dialog.As a result,this placeholder question is automatically prevented from being chosen as a test question.
(Essay)
4.9/5  (36)
(36)
Consider the following energy diagram for an enzyme-catalyzed reaction. 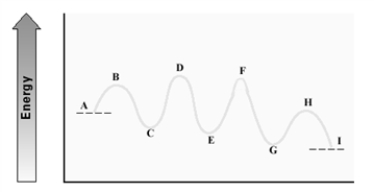 Which step has the least energetic transition state?
Which step has the least energetic transition state?
(Multiple Choice)
4.9/5  (32)
(32)
Consider the following process. 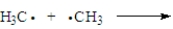 Which of the following correctly applies?
Which of the following correctly applies?
(Multiple Choice)
4.9/5  (34)
(34)
The enzyme aconitase catalyzes the hydration of the alkene functional group of aconitic acid to give two products,citric acid and isocitric acid.Isocitric acid is optically active;citric acid is not optically active.Based on your knowledge of alkene hydration and optical activity,the structure of citric acid is: 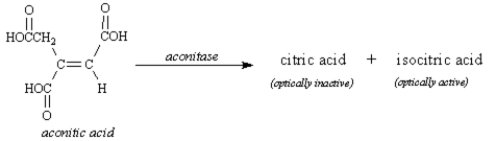
(Multiple Choice)
4.8/5  (38)
(38)
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
 -Refer to Exhibit 6-10.Using the bond dissociations values in the table below,calculate the ΔH° for the reaction in Diagram 2.Show your calculations for full credit.
-Refer to Exhibit 6-10.Using the bond dissociations values in the table below,calculate the ΔH° for the reaction in Diagram 2.Show your calculations for full credit.

(Essay)
4.8/5  (41)
(41)
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
 -Refer to Exhibit 6-10.In Diagram 2,draw arrows on the structures showing electron flow.
-Refer to Exhibit 6-10.In Diagram 2,draw arrows on the structures showing electron flow.
(Essay)
4.8/5  (37)
(37)
Exhibit 6-9
Use the reaction energy diagram below to answer the following question(s).
 -Refer to Exhibit 6-9.The free-energy change for the reaction is indicated at point _____ on the diagram.
-Refer to Exhibit 6-9.The free-energy change for the reaction is indicated at point _____ on the diagram.
(Short Answer)
4.8/5  (30)
(30)
Exhibit 6-10
Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).

Diagram 1: The first step of this reaction is shown below.
Diagram 1: The first step of this reaction is shown below.
 Diagram 2: The second and third steps of the reaction are shown below.
Diagram 2: The second and third steps of the reaction are shown below.
 -Refer to Exhibit 6-10.This reaction is an example of:
-Refer to Exhibit 6-10.This reaction is an example of:
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following compounds would produce a single product when treated with Cl2 in the presence of ultraviolet radiation?
(Multiple Choice)
4.8/5  (41)
(41)
Exhibit 6-7
Consider this reaction when answering the following question(s):
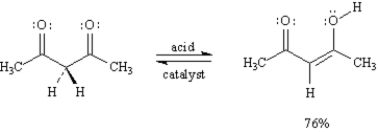 -Refer to Exhibit 6-7.
A)The structures below show the step-wise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.
-Refer to Exhibit 6-7.
A)The structures below show the step-wise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.
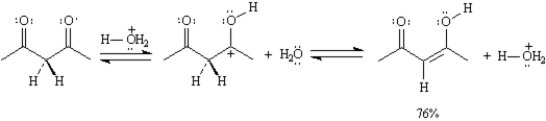 B)Calculate Keq for the reaction in part
C)Calculate ΔG° for the reaction in part
B)Calculate Keq for the reaction in part
C)Calculate ΔG° for the reaction in part
(Essay)
4.7/5  (35)
(35)
Showing 1 - 20 of 42
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)