Multiple Choice
What is the structural relationship between the two molecule shown below? 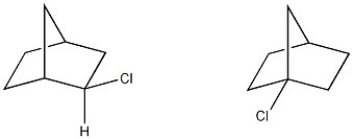
A) constitutional isomers
B) enantiomers
C) diastereomers
D) conformational isomers
E) not isomers
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Label each asymmetric carbon in the compound
Q56: The boiling point of S-2-butanol is 99°C.
Q57: How many enantiomers are there of the
Q58: How many diastereomers are there of the
Q59: How many asymmetric carbon atoms are present
Q61: How many asymmetric carbons are present in
Q62: Draw the structure of (2R,3S)-2,3-dichloropentane. Take particular
Q63: A student measured the optical activity of
Q64: Consider the four substituents below. Order them
Q65: Captopril is used to treat high blood